Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence
- PMID: 31474573
- PMCID: PMC6863385
- DOI: 10.1016/j.molcel.2019.08.005
Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence
Abstract
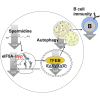
Failure to make adaptive immune responses is a hallmark of aging. Reduced B cell function leads to poor vaccination efficacy and a high prevalence of infections in the elderly. Here we show that reduced autophagy is a central molecular mechanism underlying immune senescence. Autophagy levels are specifically reduced in mature lymphocytes, leading to compromised memory B cell responses in old individuals. Spermidine, an endogenous polyamine metabolite, induces autophagy in vivo and rejuvenates memory B cell responses. Mechanistically, spermidine post-translationally modifies the translation factor eIF5A, which is essential for the synthesis of the autophagy transcription factor TFEB. Spermidine is depleted in the elderly, leading to reduced TFEB expression and autophagy. Spermidine supplementation restored this pathway and improved the responses of old human B cells. Taken together, our results reveal an unexpected autophagy regulatory mechanism mediated by eIF5A at the translational level, which can be harnessed to reverse immune senescence in humans.
Keywords: B cell; TFEB; aging; autophagy; eIF5A; spermidine.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
A.B. is a co-founder of CASMA Therapeutics.
Figures







Comment in
-
Polyamines and Aging: A CLEAR Connection?Mol Cell. 2019 Oct 3;76(1):5-7. doi: 10.1016/j.molcel.2019.09.003. Mol Cell. 2019. PMID: 31585103
-
The curious case of polyamines: spermidine drives reversal of B cell senescence.Autophagy. 2020 Mar;16(3):389-390. doi: 10.1080/15548627.2019.1698210. Epub 2019 Dec 3. Autophagy. 2020. PMID: 31795807 Free PMC article.
References
-
- Cambridge S.B., Gnad F., Nguyen C., Bermejo J.L., Krüger M., Mann M. Systems-wide proteomic analysis in mammalian cells reveals conserved, functional protein turnover. J. Proteome Res. 2011;10:5275–5284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

