Signal transducer and activator of transcription-3 drives the high-fat diet-associated prostate cancer growth
- PMID: 31474764
- PMCID: PMC6717738
- DOI: 10.1038/s41419-019-1842-4
Signal transducer and activator of transcription-3 drives the high-fat diet-associated prostate cancer growth
Abstract
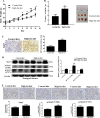
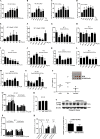
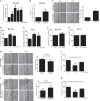
Prostate cancer (PCa) is the second leading cause of cancer death in men. PCa progression can be associated with obesity. Signal transducer and activator of transcription-3 (STAT3) plays a crucial role in PCa growth. However, whether STAT3 plays a role in high-fat diet (HFD)-associated PCa growth is unknown. Our data show that HFD feeding increases tumor size, STAT3 phosphorylation, and palmitic acid (PA) level in the xenograft tissues of the PCa-bearing xenograft mouse model. In vitro studies show that PA increases STAT3 expression and phosphorylation (STAT3-Y705) in PCa. Computational modeling suggests strong and stable binding between PA and unphosphorylated STAT3 at R593 and N538. The binding changes STAT3 structure and activity. Functional studies show that both STAT3 mutants (R583A and N538A) and STAT3 dominant negative significantly reduce PA-enhanced STAT3 phosphorylation, PA-increased PCa cell proliferation, migration, and invasion. In the xenograft mouse models, the HFD-increased tumor growth and STAT3 phosphorylation in tumors are reversed by STAT3 inhibition. Our study not only demonstrates the regulatory role of PA/STAT3 axis in HFD-associated PCa growth but also suggests a novel mechanism of how STAT3 is activated by PA. Our data suggest STAT3 as a therapeutic target for the treatment of HFD-associated PCa.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Diet-induced macrophage inhibitory cytokine 1 promotes prostate cancer progression.Endocr Relat Cancer. 2013 Dec 16;21(1):39-50. doi: 10.1530/ERC-13-0227. Print 2014 Feb. Endocr Relat Cancer. 2013. PMID: 24344250
-
The STAT3 Inhibitor Galiellalactone Effectively Reduces Tumor Growth and Metastatic Spread in an Orthotopic Xenograft Mouse Model of Prostate Cancer.Eur Urol. 2016 Mar;69(3):400-4. doi: 10.1016/j.eururo.2015.06.016. Epub 2015 Jul 2. Eur Urol. 2016. PMID: 26144873
-
Altered miRNA expression in high-fat diet-induced prostate cancer progression.Carcinogenesis. 2016 Dec;37(12):1129-1137. doi: 10.1093/carcin/bgw108. Epub 2016 Oct 7. Carcinogenesis. 2016. PMID: 27915273
-
ASC-J9® suppresses prostate cancer cell invasion via altering the sumoylation-phosphorylation of STAT3.Cancer Lett. 2018 Jul 1;425:21-30. doi: 10.1016/j.canlet.2018.02.007. Epub 2018 Feb 6. Cancer Lett. 2018. PMID: 29425687
-
An overview of the latest in state-of-the-art murine models for prostate cancer.Expert Opin Drug Discov. 2021 Nov;16(11):1349-1364. doi: 10.1080/17460441.2021.1943354. Epub 2021 Jul 5. Expert Opin Drug Discov. 2021. PMID: 34224283 Review.
Cited by
-
Histone methyltransferase KMT2D mediated lipid metabolism via peroxisome proliferator-activated receptor gamma in prostate cancer.Transl Cancer Res. 2022 Aug;11(8):2607-2621. doi: 10.21037/tcr-22-431. Transl Cancer Res. 2022. PMID: 36093518 Free PMC article.
-
Targeting lipid metabolism in metastatic prostate cancer.Ther Adv Med Oncol. 2023 Jan 30;15:17588359231152839. doi: 10.1177/17588359231152839. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 36743527 Free PMC article. Review.
-
High-fat diet feeding and palmitic acid increase CRC growth in β2AR-dependent manner.Cell Death Dis. 2019 Sep 26;10(10):711. doi: 10.1038/s41419-019-1958-6. Cell Death Dis. 2019. PMID: 31558710 Free PMC article.
-
Influence of Diet and Nutrition on Prostate Cancer.Int J Mol Sci. 2020 Feb 20;21(4):1447. doi: 10.3390/ijms21041447. Int J Mol Sci. 2020. PMID: 32093338 Free PMC article. Review.
-
Thermal proteome profiling and proteome analysis using high-definition mass spectrometry demonstrate modulation of cholesterol biosynthesis by next-generation galeterone analog VNPP433-3β in castration-resistant prostate cancer.Mol Oncol. 2025 Aug;19(8):2292-2309. doi: 10.1002/1878-0261.70009. Epub 2025 Feb 26. Mol Oncol. 2025. PMID: 40007440 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

