ALWPs Improve Cognitive Function and Regulate Aβ Plaque and Tau Hyperphosphorylation in a Mouse Model of Alzheimer's Disease
- PMID: 31474828
- PMCID: PMC6707392
- DOI: 10.3389/fnmol.2019.00192
ALWPs Improve Cognitive Function and Regulate Aβ Plaque and Tau Hyperphosphorylation in a Mouse Model of Alzheimer's Disease
Abstract
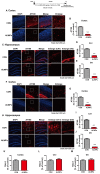
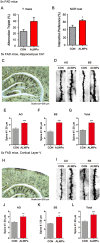
Recently, we reported that ALWPs, which we developed by combining Liuwei Dihuang pills (LWPs) with antler, regulate the LPS-induced neuroinflammatory response and rescue LPS-induced short- and long-term memory impairment in wild-type (WT) mice. In the present study, we examined the effects of ALWPs on Alzheimer's disease (AD) pathology and cognitive function in WT mice as well as 5x FAD mice (a mouse model of AD). We found that administration of ALWPs significantly reduced amyloid plaque levels in 5x FAD mice and significantly decreased amyloid β (Aβ) levels in amyloid precursor protein (APP)-overexpressing H4 cells. In addition, ALWPs administration significantly suppressed tau hyperphosphorylation in 5x FAD mice. Oral administration of ALWPs significantly improved long-term memory in scopolamine (SCO)-injected WT mice and 5x FAD mice by altering dendritic spine density. Importantly, ALWPs promoted spinogenesis in primary hippocampal neurons and WT mice and modulated the dendritic spine number in an extracellular signal-regulated kinase (ERK)-dependent manner. Taken together, our results suggest that ALWPs are a candidate therapeutic drug for AD that can modulate amyloid plaque load, tau phosphorylation, and synaptic/cognitive function.
Keywords: Alzheimer’s disease; Aβ; amyloid plaque; dendritic spines; long-term memory; tau.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

