Testing the Translational Power of the Zebrafish: An Interspecies Analysis of Responses to Cardiovascular Drugs
- PMID: 31474857
- PMCID: PMC6707810
- DOI: 10.3389/fphar.2019.00893
Testing the Translational Power of the Zebrafish: An Interspecies Analysis of Responses to Cardiovascular Drugs
Abstract
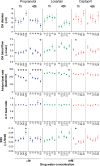
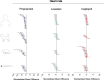
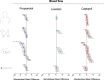
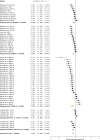
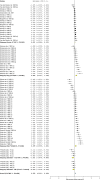
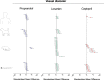
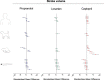
The zebrafish is rapidly emerging as a promising alternative in vivo model for the detection of drug-induced cardiovascular effects. Despite its increasing popularity, the ability of this model to inform the drug development process is often limited by the uncertainties around the quantitative relevance of zebrafish responses compared with nonclinical mammalian species and ultimately humans. In this test of concept study, we provide a comparative quantitative analysis of the in vivo cardiovascular responses of zebrafish, rat, dog, and human to three model compounds (propranolol, losartan, and captopril), which act as modulators of two key systems (beta-adrenergic and renin-angiotensin systems) involved in the regulation of cardiovascular functions. We used in vivo imaging techniques to generate novel experimental data of drug-mediated cardiovascular effects in zebrafish larvae. These data were combined with a database of interspecies mammalian responses (i.e., heart rate, blood flow, vessel diameter, and stroke volume) extracted from the literature to perform a meta-analysis of effect size and direction across multiple species. In spite of the high heterogeneity of study design parameters, our analysis highlighted that zebrafish and human responses were largely comparable in >80% of drug/endpoint combinations. However, it also revealed a high intraspecies variability, which, in some cases, prevented a conclusive interpretation of the drug-induced effect. Despite the shortcomings of our study, the meta-analysis approach, combined with a suitable data visualization strategy, enabled us to observe patterns of response that would likely remain undetected with more traditional methods of qualitative comparative analysis. We propose that expanding this approach to larger datasets encompassing multiple drugs and modes of action would enable a rigorous and systematic assessment of the applicability domain of the zebrafish from both a mechanistic and phenotypic standpoint. This will increase the confidence in its application for the early detection of adverse drug reactions in any major organ system.
Keywords: beta-adrenergic receptor; cardiovascular effects; comparative pharmacology; drug safety; meta-analysis; preclinical species; renin–angiotensin system; zebrafish.
Figures



Similar articles
-
A multi-endpoint in vivo larval zebrafish (Danio rerio) model for the assessment of integrated cardiovascular function.J Pharmacol Toxicol Methods. 2014 Jan-Feb;69(1):30-8. doi: 10.1016/j.vascn.2013.10.002. Epub 2013 Oct 16. J Pharmacol Toxicol Methods. 2014. PMID: 24140389
-
Comparative studies on differential inhibition of the renin - angiotensin system in the anesthetized guinea pig.Can J Physiol Pharmacol. 1995 Oct;73(10):1512-8. doi: 10.1139/y95-209. Can J Physiol Pharmacol. 1995. PMID: 8748944
-
Comparison of Zebrafish Larvae and hiPSC Cardiomyocytes for Predicting Drug-Induced Cardiotoxicity in Humans.Toxicol Sci. 2019 Oct 1;171(2):283-295. doi: 10.1093/toxsci/kfz165. Toxicol Sci. 2019. PMID: 31359052 Free PMC article.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
The preclinical basis of the therapeutic evaluation of losartan.J Hypertens Suppl. 1995 Jul;13(1):S1-13. doi: 10.1097/00004872-199507001-00001. J Hypertens Suppl. 1995. PMID: 18800450 Review.
Cited by
-
Arrhythmic Effects Evaluated on Caenorhabditis elegans: The Case of Polypyrrole Nanoparticles.ACS Nano. 2023 Sep 12;17(17):17273-17284. doi: 10.1021/acsnano.3c05245. Epub 2023 Aug 25. ACS Nano. 2023. PMID: 37624669 Free PMC article.
-
Studies in Zebrafish Demonstrate That CNNM2 and NT5C2 Are Most Likely the Causal Genes at the Blood Pressure-Associated Locus on Human Chromosome 10q24.32.Front Cardiovasc Med. 2020 Sep 2;7:135. doi: 10.3389/fcvm.2020.00135. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32984406 Free PMC article.
-
Zebrafish drug screening identifies candidate therapies for neuroprotection after spontaneous intracerebral haemorrhage.Dis Model Mech. 2022 Mar 1;15(3):dmm049227. doi: 10.1242/dmm.049227. Epub 2022 Mar 29. Dis Model Mech. 2022. PMID: 35098999 Free PMC article.
-
Ecklonia cava Extract and Its Derivative Dieckol Promote Vasodilation by Modulating Calcium Signaling and PI3K/AKT/eNOS Pathway in In Vitro and In Vivo Models.Biomedicines. 2021 Apr 19;9(4):438. doi: 10.3390/biomedicines9040438. Biomedicines. 2021. PMID: 33921856 Free PMC article.
-
Diphlorethohydroxycarmalol Isolated from Ishige okamurae Exerts Vasodilatory Effects via Calcium Signaling and PI3K/Akt/eNOS Pathway.Int J Mol Sci. 2021 Feb 5;22(4):1610. doi: 10.3390/ijms22041610. Int J Mol Sci. 2021. PMID: 33562632 Free PMC article.
References
-
- Altimiras J., Aissaoui A., Tort L. (1995). Is the short-term modulation of heart rate in teleost fish physiologically significant? Assessment by spectral analysis techniques. Braz. J. Med. Biol. Res. 28, 1197–1206. - PubMed
LinkOut - more resources
Full Text Sources

