Congenital Transmission of Trypanosoma cruzi: A Review About the Interactions Between the Parasite, the Placenta, the Maternal and the Fetal/Neonatal Immune Responses
- PMID: 31474955
- PMCID: PMC6702454
- DOI: 10.3389/fmicb.2019.01854
Congenital Transmission of Trypanosoma cruzi: A Review About the Interactions Between the Parasite, the Placenta, the Maternal and the Fetal/Neonatal Immune Responses
Abstract
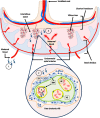
Chagas disease (CD), caused by the protozoan parasite Trypanosoma cruzi, is considered a neglected tropical disease by the World Health Organization. Congenital transmission of CD is an increasingly relevant public health problem. It progressively becomes the main transmission route over others and can occur in both endemic and non-endemic countries. Though most congenitally infected newborns are asymptomatic at birth, they display higher frequencies of prematurity, low birth weight, and lower Apgar scores compared to uninfected ones, and some suffer from severe symptoms. If not diagnosed and treated, infected newborns are at risk of developing disabling and life-threatening chronic pathologies later in life. The success or failure of congenital transmission depends on interactions between the parasite, the placenta, the mother, and the fetus. We review and discuss here the current knowledge about these parameters, including parasite virulence factors such as exovesicles, placental tropism, potential placental defense mechanisms, the placental transcriptome of infected women, gene polymorphism, and the maternal and fetal/neonatal immune responses, that might modulate the risk of T. cruzi congenital transmission.
Keywords: Trypanosoma cruzi; congenital chagas disease; infection; maternal-fetal interactions; placenta.
Figures
References
-
- Almeida I. C., Gazzinelli R. T. (2001). Proinflammatory activity of glycosylphosphatidylinositol anchors derived from Trypanosoma cruzi: structural and functional analyses. J. Leukoc. Biol. 70 467–477. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

