Individual Effector/Regulator T Cell Ratios Impact Bone Regeneration
- PMID: 31475013
- PMCID: PMC6706871
- DOI: 10.3389/fimmu.2019.01954
Individual Effector/Regulator T Cell Ratios Impact Bone Regeneration
Abstract
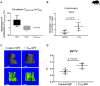
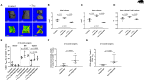
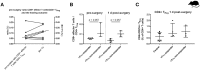
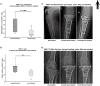
There is increasing evidence that T lymphocytes play a key role in controlling endogenous regeneration. Regeneration appears to be impaired in case of local accumulation of CD8+ effector T cells (TEFF), impairing endogenous regeneration by increasing a primary "useful" inflammation toward a damaging level. Thus, rescuing regeneration by regulating the heightened pro-inflammatory reaction employing regulatory CD4+ T (TReg) cells could represent an immunomodulatory option to enhance healing. Hypothesis was that CD4+ TReg might counteract undesired effects of CD8+ TEFF. Using adoptive TReg transfer, bone healing was consistently improved in mice possessing an inexperienced immune system with low amounts of CD8+ TEFF. In contrast, mice with an experienced immune system (high amounts of CD8+ TEFF) showed heterogeneous bone repair with regeneration being dependent upon the individual TEFF/TReg ratio. Thus, the healing outcome can only be improved by an adoptive TReg therapy, if an unfavorable TEFF/TReg ratio can be reshaped; if the individual CD8+ TEFF percentage, which is dependent on the individual immune experience can be changed toward a favorable ratio by the TReg transfer. Remarkably, also in patients with impaired fracture healing the TEFF/TReg ratio was higher compared to uneventful healers, validating our finding in the mouse osteotomy model. Our data demonstrate for the first time the key-role of a balanced TEFF/TReg response following injury needed to reach successful regeneration using bone as a model system. Considering this strategy, novel opportunities for immunotherapy in patients, which are at risk for impaired healing by targeting TEFF cells and supporting TReg cells to enhance healing are possible.
Keywords: bone healing; effector T cell; mouse model; regeneration; regulatory T (Treg) cell.
Figures


References
-
- Bucher CH, Lei H, Duda GN, Volk HD, Schmidt-Bleek K. The role of immune Reactivity in bone regeneration. In: Zorzi AR, editor. Advanced Techniques in Bone Regeneration. IntechOpen. (2016). Available online at: https://www.intechopen.com/books/advanced-techniques-in-bone-regeneratio...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

