Pathogenic effect of a TGFBR1 mutation in a family with Loeys-Dietz syndrome
- PMID: 31475485
- PMCID: PMC6785444
- DOI: 10.1002/mgg3.943
Pathogenic effect of a TGFBR1 mutation in a family with Loeys-Dietz syndrome
Abstract
Background: Thoracic aortic aneurysms and dissections (TAAD) may have a heritable cause in up to 20% of cases. We aimed to investigate the pathogenic effect of a TGFBR1 mutation in relation to TAAD.
Methods: Co-segregation analysis was performed followed by functional investigations, including myogenic transdifferentiation.
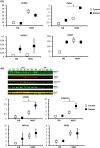
Results: The c.1043G>A TGFBR1 mutation was found in the index patient, in a deceased brother, and in five presymptomatic family members. Evidence for pathogenicity was found by the predicted damaging effect of this mutation and the co-segregation in the family. Functional analysis with myogenic transdifferentiation of dermal fibroblasts to smooth muscle-like cells, revealed increased myogenic differentiation in patient cells with the TGFBR1 mutation, shown by a higher expression of myogenic markers ACTA2, MYH11 and CNN1 compared to cells from healthy controls.
Conclusion: Our findings confirm the pathogenic effect of the TGFBR1 mutation in causing TAAD in Loeys-Dietz syndrome and show increased myogenic differentiation of patient fibroblasts.
Keywords: Loeys-Dietz syndrome; Myogenic transdifferentiation of fibroblasts; Smooth muscle-like cells; TGFBR1 mutation; Thoracic aortic aneurysm and aortic dissection.
© 2019 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
None of the authors have any conflicts of interest to report.
Figures

References
-
- Albornoz, G. , Coady, M. A. , Roberts, M. , Davies, R. R. , Tranquilli, M. , Rizzo, J. A. , & Elefteriades, J. A. (2006). Familial thoracic aortic aneurysms and dissections–incidence, modes of inheritance, and phenotypic patterns. Annals of Thoracic Surgery, 82, 1400–1405. 10.1016/j.athoracsur.2006.04.098 - DOI - PubMed
-
- Bian, Y. , Terse, A. , Du, J. , Hall, B. , Molinolo, A. , Zhang, P. , et al. (2009). Progressive tumor formation in mice with conditional deletion of TGF‐beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Research, 69, 5918–5926. 10.1158/0008-5472.CAN-08-4623 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

