Structural Origin of the Large Redox-Linked Reorganization in the 2-Oxoglutarate Dependent Oxygenase, TauD
- PMID: 31475523
- PMCID: PMC7092798
- DOI: 10.1021/jacs.9b07493
Structural Origin of the Large Redox-Linked Reorganization in the 2-Oxoglutarate Dependent Oxygenase, TauD
Abstract
2-Oxoglutarate (2OG)-dependent oxygenases catalyze a wide range of chemical transformations via C-H bond activation. Prior studies raised the question of whether substrate hydroxylation by these enzymes occurs via a hydroxyl rebound or alkoxide mechanism and highlighted the need to understand the thermodynamic properties of transient intermediates. A recent spectroelectrochemical investigation of the 2OG-dependent oxygenase, taurine hydroxylase (TauD), revealed a strong link between the redox potential of the Fe(II)/Fe(III) couple and conformational changes of the enzyme. In this study, we show that the redox potential of wild-type TauD varies by 468 mV between the reduction of 2OG-Fe(III)-TauD (-272 mV) and oxidation of 2OG-Fe(II)-TauD (+196 mV). We use active site variants to investigate the structural origin of the redox-linked reorganization and the contributions of the metal-bound residues to the dynamic tuning of the redox potential of TauD. Time-dependent redox titrations show that reorganization occurs as a multistep process. Transient optical absorption and infrared spectroelectrochemistry show that substitution of any metal ligand alters the kinetics and thermodynamics of the reorganization. The H99A variant shows the largest net redox change relative to the wild-type protein, suggesting that redox-coupled protonation of H99 is required for high redox potentials of the metal. The D101Q and H255Q variants also suppress the conformational change, supporting their involvement in the structural rearrangement. Similar redox-linked conformational changes are observed in another 2OG dependent oxygenase, ethylene-forming enzyme, indicating that dynamic structural flexibility and the associated thermodynamic tuning may be a common phenomenon in this family of enzymes.
Figures






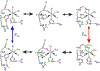
References
-
- Proshlyakov DA; Hausinger RP Transient Iron Species in the Catalytic Mechanism of the Archetypal α-Ketoglutarate-Dependent Dioxygenase, TauD In Iron-Containing Enzymes: Versatile Catalysts of Hydroxylation Reactions in Nature; Visser SP, De Kumar D, Eds.; Royal Society of Chemistry: Cambridge, UK, 2012, pp 67–87.
-
- Eichhorn E; Van Der Ploeg JR; Kertesz MA; Leisinger T Characterization of α-Ketoglutarate-Dependent Taurine Dioxygenase from Escherichia coli. J. Biol. Chem 1997, 272, 23031–23036. - PubMed
-
- Meunier B; de Visser SP; Shaik S Mechanism of Oxidation Reactions Catalyzed by Cytochrome P450 Enzymes. Chem. Rev 2004, 104, 3947–3980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

