Crystal structure of TchmY from Actinoplanes teichomyceticus
- PMID: 31475923
- PMCID: PMC6718148
- DOI: 10.1107/S2053230X19010914
Crystal structure of TchmY from Actinoplanes teichomyceticus
Abstract
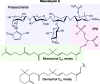
Moenomycin-type antibiotics are phosphoglycolipids that are notable for their unique modes of action and have proven to be useful in animal nutrition. The gene clusters tchm from Actinoplanes teichomyceticus and moe from Streptomyces are among a limited number of known moenomycin-biosynthetic pathways. Most genes in tchm have counterparts in the moe cluster, except for tchmy and tchmz, the functions of which remain unknown. Sequence analysis indicates that TchmY belongs to the isoprenoid enzyme C2-like superfamily and may serve as a prenylcyclase. The enzyme was proposed to be involved in terminal cyclization of the moenocinyl chain in teichomycin, leading to the diumycinol chain of moenomycin isomers. Here, recombinant TchmY protein was expressed in Escherichia coli and its crystal structure was solved by SIRAS. Structural analysis and comparison with other prenylcyclases were performed. The overall fold of TchmY consists of an (α/α)6-barrel, and a potential substrate-binding pocket is found in the central chamber. These results should provide important information regarding the biosynthetic basis of moenomycin antibiotics.
Keywords: Actinoplanes teichomyceticus; crystal structure; prenylcyclases.
Figures



References
-
- Adams, P. D., Afonine, P. V., Bunkóczi, G., Chen, V. B., Davis, I. W., Echols, N., Headd, J. J., Hung, L.-W., Kapral, G. J., Grosse-Kunstleve, R. W., McCoy, A. J., Moriarty, N. W., Oeffner, R., Read, R. J., Richardson, D. C., Richardson, J. S., Terwilliger, T. C. & Zwart, P. H. (2010). Acta Cryst. D66, 213–221. - PMC - PubMed
-
- Bauer, F. & Dost, G. (1965). Antimicrob. Agents Chemother. 5, 749–752. - PubMed
-
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. - PubMed
-
- Chan, H.-C., Ko, T.-P., Huang, C.-H. & Guo, R.-T. (2015). ChemBioEng Rev. 2, 133–140.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

