Bronchodilation induced by PGE2 is impaired in Group III pulmonary hypertension
- PMID: 31476020
- PMCID: PMC6976782
- DOI: 10.1111/bph.14854
Bronchodilation induced by PGE2 is impaired in Group III pulmonary hypertension
Abstract
Background and purpose: In patients with pulmonary hypertension (PH) associated with lung disease and/or hypoxia (Group III), decreased pulmonary vascular tone and tissue hypoxia is therapeutically beneficial. PGE2 and PGI2 induce potent relaxation of human bronchi from non-PH (control) patients via EP4 and IP receptors, respectively. However, the effects of PGE2 /PGI2 and their mimetics on human bronchi from PH patients are unknown. Here, we have compared relaxant effects of several PGI2 -mimetics approved for treating PH Group I with several PGE2 -mimetics, in bronchial preparations derived from PH Group III and control patients.
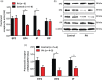
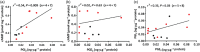
Experimental approach: Relaxation of bronchial muscle was assessed in samples isolated from control and PH Group III patients. Expression of prostanoid receptors was analysed by western blot and real-time PCR, and endogenous PGE2 , PGI2 , and cAMP levels were determined by ELISA.
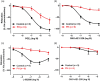
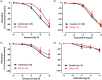
Key results: Maximal relaxations induced by different EP4 receptor agonists (PGE2 , L-902688, and ONO-AE1-329) were decreased in human bronchi from PH patients, compared with controls. However, maximal relaxations produced by PGI2 -mimetics (iloprost, treprostinil, and beraprost) were similar for both groups of patients. Both EP4 and IP receptor protein and mRNA expressions were significantly lower in human bronchi from PH patients. cAMP levels significantly correlated with PGI2 but not with PGE2 levels.
Conclusion and implications: The PGI2 -mimetics retained maximal bronchodilation in PH Group III patients, whereas bronchodilation induced by EP4 receptor agonists was decreased. Restoration of EP4 receptor expression in airways of PH Group III patients with respiratory diseases could bring additional therapeutic benefit.
© 2019 The British Pharmacological Society.
Conflict of interest statement
This work was funded by an educational research grant from United Therapeutics to X.N. L.H.C. has received educational research grants from United Therapeutics and honoraria UTC. A.M.S. and A.C.N. are employees of United Therapeutics.
Figures

References
-
- Abramovitz, M. , Adam, M. , Boie, Y. , Carriere, M. , Denis, D. , Godbout, C. , … Metters, K. M. (2000). The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochimica et Biophysica Acta, 1483, 285–293. 10.1016/S1388-1981(99)00164-X - DOI - PubMed
-
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 - DOI - PMC - PubMed
-
- Ali, F. Y. , Egan, K. , FitzGerald, G. A. , Desvergne, B. , Wahli, W. , Bishop‐Bailey, D. , … Mitchell, J. A. (2006). Role of prostacyclin versus peroxisome proliferator‐activated receptor beta receptors in prostacyclin sensing by lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology, 34, 242–246. 10.1165/rcmb.2005-0289OC - DOI - PubMed
-
- Aso, H. , Ito, S. , Mori, A. , Suganuma, N. , Morioka, M. , Takahara, N. , … Hasegawa, Y. (2013). Differential regulation of airway smooth muscle cell migration by E‐prostanoid receptor subtypes. American Journal of Respiratory Cell and Molecular Biology, 48, 322–329. 10.1165/rcmb.2012-0158OC - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

