Taxanes for adjuvant treatment of early breast cancer
- PMID: 31476253
- PMCID: PMC6718224
- DOI: 10.1002/14651858.CD004421.pub3
Taxanes for adjuvant treatment of early breast cancer
Abstract
Background: Adjuvant chemotherapy improves survival in premenopausal and postmenopausal women with early breast cancer. Taxanes are highly active chemotherapy agents used in metastatic breast cancer. Review authors examined their role in early breast cancer. This review is an update of a Cochrane Review first published in 2007.
Objectives: To assess the effects of taxane-containing adjuvant chemotherapy regimens for treatment of women with operable early breast cancer.
Search methods: For this review update, we searched the Specialised Register of the Cochrane Breast Cancer Group, MEDLINE, Embase, CENTRAL (2018, Issue 6), the WHO International Clinical Trials Registry Platform (ICTRP), and ClinicalTrials.gov on 16 July 2018, using key words such as 'early breast cancer' and 'taxanes'. We screened reference lists of other related literature reviews and articles, contacted trial authors, and applied no language restrictions.
Selection criteria: Randomised trials comparing taxane-containing regimens versus non-taxane-containing regimens in women with operable breast cancer were included. Studies of women receiving neoadjuvant chemotherapy were excluded.
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias and quality of the evidence using the GRADE approach. Hazard ratios (HRs) were derived for time-to-event outcomes, and meta-analysis was performed using a fixed-effect model. The primary outcome measure was overall survival (OS); disease-free survival (DFS) was a secondary outcome measure. Toxicity was represented as odds ratios (ORs), and quality of life (QoL) data were extracted when present.
Main results: This review included 29 studies (27 full-text publications and 2 abstracts or online theses). The updated analysis included 41,911 randomised women; the original review included 21,191 women. Taxane-containing regimens improved OS (HR 0.87, 95% confidence interval (CI) 0.83 to 0.92; high-certainty evidence; 27 studies; 39,180 women; 6501 deaths) and DFS (HR, 0.88, 95% CI 0.85 to 0.92; high-certainty evidence; 29 studies; 41,909 women; 10,271 reported events) compared to chemotherapy without a taxane. There was moderate to substantial heterogeneity across studies for OS and DFS (respectively).When a taxane-containing regimen was compared with the same regimen without a taxane, the beneficial effects of taxanes persisted for OS (HR 0.84, 95% CI 0.77 to 0.92; P < 0.001; 7 studies; 10,842 women) and for DFS (HR 0.84, 95% CI 0.78 to 0.90; P < 0.001; 7 studies; 10,842 women). When a taxane-containing regimen was compared with the same regimen with another drug or drugs that were substituted for the taxane, a beneficial effect was observed for OS and DFS with the taxane-containing regimen (OS: HR 0.80, 95% CI 0.74 to 0.86; P < 0.001; 13 studies; 16,196 women; DFS: HR 0.83, 95% CI 0.78 to 0.88; P < 0.001; 14 studies; 16,823 women). Preliminary subgroup analysis by lymph node status showed a survival benefit with taxane-containing regimens in studies of women with lymph node-positive disease only (HR 0.83, 95% CI 0.78 to 0.88; P < 0.001; 17 studies; 22,055 women) but less benefit in studies of women both with and without lymph node metastases or with no lymph node metastases. Taxane-containing regimens also improved DFS in women with lymph node-positive disease (HR 0.84, 95% CI 0.80 to 0.88; P < 0.001; 17 studies; 22,055 women), although the benefit was marginal in studies of women both with and without lymph node-positive disease (HR 0.95, 95% CI 0.88 to 1.02; 9 studies; 12,998 women) and was not apparent in studies of women with lymph node-negative disease (HR 0.99, 95% CI 0.86 to 1.14; 3 studies; 6856 women).Taxanes probably result in a small increase in risk of febrile neutropenia (odds ratio (OR) 1.55, 95% CI 0.96 to 2.49; moderate-certainty evidence; 24 studies; 33,763 women) and likely lead to a large increase in grade 3/4 neuropathy (OR 6.89, 95% CI 3.23 to 14.71; P < 0.001; moderate-certainty evidence; 22 studies; 31,033 women). Taxanes probably cause little or no difference in cardiotoxicity compared to regimens without a taxane (OR 0.87, 95% CI 0.56 to 1.33; moderate-certainty evidence; 23 studies; 32,894 women). Seven studies reported low-quality evidence for QoL; overall, taxanes may make little or no difference in QoL compared to chemotherapy without a taxane during the follow-up period; however, the duration of follow-up differed across studies. Only one study, which was conducted in Europe, provided cost-effectiveness data.
Authors' conclusions: This review of studies supports the use of taxane-containing adjuvant chemotherapy regimens, with improvement in overall survival and disease-free survival for women with operable early breast cancer. This benefit persisted when analyses strictly compared a taxane-containing regimen versus the same regimen without a taxane or the same regimen with another drug that was substituted for the taxane. Preliminary evidence suggests that taxanes are more effective for women with lymph node-positive disease than for those with lymph node-negative disease. Considerable heterogeneity across studies probably reflects the varying efficacy of the chemotherapy backbones of the comparator regimens used in these studies. This review update reports results that are remarkably consistent with those of the original review, and it is highly unlikely that this review will be updated, as new trials are assessing treatments based on more detailed breast cancer biology.
Conflict of interest statement
MW: none known. LB: none known. TF: none known. DG: none known. AN: no relevant conflicts of interest relevant to the topic under review, breast cancer. All declarations relate directly to consulting work and clinical trials in relation to malignant mesothelioma or brain cancer. NW: intermittently served on advisory boards for pharmaceutical companies and been paid honoraria for educational lectures sponsored by pharmaceutical companies though not related to the topic under review.
Figures






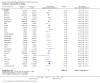



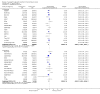











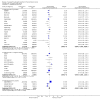



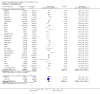











Update of
-
Taxanes for adjuvant treatment of early breast cancer.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD004421. doi: 10.1002/14651858.CD004421.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2019 Sep 02;9:CD004421. doi: 10.1002/14651858.CD004421.pub3. PMID: 17943815 Updated.
References
References to studies included in this review
ADEBAR {published data only}
-
- Gauger K, Bismarck FV, Heinrigs M, Janni W, Steinfeld, Augustin D, et al. Phase III study evaluating the role of docetaxel in the adjuvant setting of breast cancer patients with ≥ 4 involved lymph nodes: ADEBAR‐Study. Journal of Clinical Oncology 2005;23(Suppl 16):908.
-
- Janni W, Harbeck N, Sommer H, Rack B, Augustin D, Jueckstock J, et al. Randomized phase III trial of fluorouracil/epirubicin/cyclophosphamide (FEC) versus epirubicin/cyclophosphamide/docetaxel in patients with node positive primary breast cancer: final analysis of the ADEBAR study. Author provided draft manuscript. Received on the 10th of March 2014.
-
- Janni W, Harbeck N, Sommer H, Rack B, Augustin D, Simon W, et al. Sequential treatment with epirubicin/cyclophosphamide followed by docetaxel is equieffective, but less toxic than FEC120, in the adjuvant treatment of breast cancer patients with extensive lymph node involvement: the German ADEBAR phase III study. Cancer Research 2009;69:604.
-
- Janni W, Harbeck N, Sommer H, Rack B, Augustin D, Simon W, et al. Sequential treatment with epirubicin/cyclophosphamide followed by docetaxel versus FEC120 in the adjuvant treatment of node‐positive breast cancer patients: final survival analysis of the German ADEBAR phase III study. Journal of Clinical Oncology 2012;30:1081.
BCIRG 001 {published data only}
-
- Mackey JR, Martin M, Pienkowski T, Rolski J, Guastalla JP, Sami A, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node‐positive breast cancer: 10‐year follow‐up of the phase 3 randomised BCIRG 001 trial. Lancet Oncology 2013;14(1):72‐80. - PubMed
-
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node‐positive breast cancer. New England Journal of Medicine 2005;352(22):2302‐13. - PubMed
BIG 2‐98 {published data only}
-
- Crown JP, Francis P, Leo A. Docetaxel given concurrently with or sequentially to anthracycline‐based adjuvant therapy for patients with node‐positive breast cancer, in comparison with non‐taxane combination chemotherapy: first results of the BIG 2‐98 trial at 5 years median follow‐up. Journal of Clinical Oncology 2006;24(Suppl 18):LBA519.
-
- Francis P, Crown J, Leo A, Buyse M, Balil A, Andersson M, et al. Adjuvant chemotherapy with sequential or concurrent anthracycline and docetaxel: Breast International Group 02‐98 randomized trial. Journal of the National Cancer Institute 2008;100(2):121‐33. - PubMed
-
- Sonnenblick A, Francis PA, Azim Jr HA, Azambuja E, Nordenskjöld B, Gutiérez J, et al. Final 10‐year results of the Breast International Group 2‐98 phase III trial and the role of Ki67 in predicting benefit of adjuvant docetaxel in patients with oestrogen receptor positive breast cancer. European Journal of Cancer 2015;51(12):1481‐9. - PubMed
Boccardo {published data only}
-
- Boccardo F, Amadori D, Guglielmini P, Sismondi P, Farris A, Agostara B, et al. Epirubicin followed by cyclophosphamide, methotrexate and 5‐fluorouracil versus paclitaxel followed by epirubicin and vinorelbine in patients with high‐risk operable breast cancer. Oncology (United States) 2010;78(3‐4):274‐81. - PubMed
CALGB 40101 {published data only}
-
- Shulman LN, Berry DA, Cirrincione CT, Becker H, Perez EA, O'Regan R, et al. Comparison of doxorubicin and cyclophosphamide (AC) versus single‐agent paclitaxel (T) as adjuvant therapy for breast cancer in women with 0‐3 positive axillary nodes: CALGB 40101. Journal of Clinical Oncology 2013;31:1007. - PMC - PubMed
-
- Shulman LN, Berry DA, Cirrincione CT, Becker HP, Perez EA, O'Regan R, et al. Comparison of doxorubicin and cyclophosphamide versus single‐agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary notes: GALGB40101 (Alliance). Journal of Clinical Oncology 2014;32(22):2311‐7. - PMC - PubMed
-
- Shulman LN, Cirrincione CT, Berry DA, Becker HP, Perez EA, O'Regan R, et al. Six cycles of doxorubicin and cyclophosphamide or paclitaxel are not superior to four cycles as adjuvant chemotherapy for breast cancer in women with zero to three positive axillary nodes: Cancer and Leukemia Group B 40101. Journal of Clinical Oncology 2012;30(33):4071‐6. - PMC - PubMed
CALGB 9344 {published data only}
-
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node‐positive primary breast cancer. Journal of Clinical Oncology March 2003;21(6):976‐83. - PubMed
DEVA {published data only}
-
- Coombes RC, Bliss JM, Espie M, Erdkamp F, Wals J, Tres A, et al. Randomized, phase III trial of sequential epirubicin and docetaxel versus epirubicin alone in postmenopausal patients with node‐positive breast cancer. Journal of Clinical Oncology 2011;29(24):3247‐54. - PubMed
-
- Coombes RC, Bliss JM, Espie M, Erdkamp F, Wals JJ, Tres A, et al. DEVA: randomized trial of sequential epirubicin and docetaxel versus epirubicin alone in node‐positive postmenopausal early breast cancer (EBC) patients. Journal of Clinical Oncology 2010;28:15. - PubMed
-
- International Cancer Collaborative Group. A multicentre randomised trial of sequential epirubicin and docetaxel versus epirubicin in node positive postmenopausal breast cancer patients. Protocol only 1997.
E2197 {published data only}
-
- Goldstein L, O'Neill A, Sparano J, et al. E2197. Phase III AT vs. AC in the adjuvant treatment of node‐positive and high‐risk node‐negative breast cancer. Journal of Clinical Oncology 2005;23(16 Suppl 7):Abstract 512.
-
- Goldstein LJ, O'Neill A, Sparano JA, Perez EA, Shulman LN, Martino S, et al. Concurrent doxorubicin plus docetaxel is not more effective than concurrent doxorubicin plus cyclophosphamide in operable breast cancer with 0 to 3 positive axillary nodes: North American Breast Cancer Intergroup Trial E 2197. Journal of Clinical Oncology 2008;26(25):4092‐9. - PMC - PubMed
-
- Sparano J, O'Neil A, Gray R, Perez E, Shulman L, Martino S, et al. 10‐year update of E2197: phase III doxorubicin/docetaxel (AT) versus doxorubicin/cyclophosphamide (AC) adjuvant treatment of LN+ and high‐risk LN‐ breast cancer and the comparison of the prognostic utility of the 21‐gene recurrence score (RS) with clinicopathologic features. Journal of Clinical Oncology 2012;30:1021.
ECTO {published data only}
-
- Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A, et al. European Cooperative Trial in Operable Breast Cancer (ECTO). Improved freedom from progression from adding paclitaxel to doxorubicin followed by cyclophosphamide methotrexate and fluorouracil. Journal of Clinical Oncology 2005;23(Suppl 7):513. - PubMed
-
- Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A, et al. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clinical Cancer Research 2005;11(24):8715‐21. - PubMed
-
- Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Lluch A, et al. Phase III trial evaluating the addition of paclitaxel to doxorubicin followed by cyclophosphamide, methotrexate, and fluorouracil, as adjuvant or primary systemic therapy: European Cooperative Trial in Operable Breast Cancer. Journal of Clinical Oncology 2009;27(15):2474‐81. - PubMed
ELDA {published data only}
-
- Nuzzo F, Morabito A, Maio E, Rella F, Gravina A, Labonia V, et al. Weekly docetaxel versus CMF as adjuvant chemotherapy for elderly breast cancer patients: safety data from the multicentre phase 3 randomised ELDA trial. Critical Reviews in Oncology/Hematology 2008;66(2):171‐80. - PubMed
-
- Perrone F, Nuzzo F, Rella F, Gravina A, Iodice G, Labonia V, et al. Weekly docetaxel versus CMF as adjuvant chemotherapy for older women with early breast cancer: final results of the randomized phase III ELDA trial. Annals of Oncology 2015;26(4):675‐82. - PubMed
FinHer {published data only}
-
- Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. Journal of Clinical Oncology 2009;27(34):5685‐92. - PubMed
-
- Joensuu H, Kellokumpu‐Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without Trastuzumab for breast cancer. New England Journal of Medicine 2006;354(8):809‐20. - PubMed
GEICAM 2003‐02 {published data only}
-
- Martin M. Fluorouracil plus doxorubicin and cyclophosphamide (FAC) versus FAC plus weekly paclitaxel as adjuvant treatment of node negative high risk breast cancer patients. Physician Data Query (PDQ) 2005.
-
- Martin M, Ruiz A, Ruiz‐Borrego M, Barnadas A, Gonzales S, Calavo L, et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high‐risk, node‐negative breast cancer: results form the GEICAM/2003‐02 study. Journal of Clinical Oncology 2013;31(20):2593‐9. - PubMed
GEICAM 9805 {published data only}
-
- Martin M, Lluch A, Segui MA, Anton A, Fernandez‐Chacon C, Ruiz A, et al. Toxicity and health‐related quality of life in node negative breast cancer patients receiving adjuvant treatment with TAC or FAC: impact of adding prophylactic growth factors to TAC. GEICAM Study 9805. Journal of Clinical Oncology 2005;23:604.
-
- Martin M, Lluch A, Segui MA, Anton A, Ruiz A, Ramos A. Prophylactic growth factor support with adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) for node‐negative breast cancer: an interim safety analysis of the GEICAM 9805 study. Journal of Clinical Oncology 2004;22:620.
-
- Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, et al. Adjuvant docetaxel for high‐risk, node‐negative breast cancer. New England Journal of Medicine 2010;363(23):2200‐10. - PubMed
-
- Martín M, Lluch A, Seguí MA, Ruiz A, Ramos M, Adrover E, et al. Toxicity and health‐related quality of life in breast cancer patients receiving adjuvant docetaxel, doxorubicin, cyclophosphamide (TAC) or 5‐fluorouracil, doxorubicin and cyclophosphamide (FAC): impact of adding primary prophylactic granulocyte‐colony stimulating factor to the TAC regimen. Annals of Oncology 2006;17(8):1205‐12. - PubMed
GEICAM 9906 {published data only}
-
- Martin M, Rodriguez‐Lescure A, Ruiz A, Alba E, Calvo L, Ruiz‐Borrego M, et al. Molecular predictors of efficacy of adjuvant weekly paclitaxel in early breast cancer. Breast Cancer Research and Treatment 2010;123(1):149‐57. - PubMed
-
- Martin M, Rodriguez‐Lescure A, Ruiz A, Alba E, Calvo L, Ruiz‐Borrego M, et al. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. Journal of the National Cancer Institute 2008;100(11):805‐14. - PubMed
-
- Martín M, Rodríguez‐Lescure A, Ruiz A, Alba E, Calvo L, Ruiz‐Borrego M, et al. Multicentre, randomized phase 3 study of adjuvant chemotherapy for node positive breast cancer comparing 6 cycles of FEC(90) versus 4 cycles of FEC(90) followed by 8 weekly paclitaxel administrations: interim efficacy analysis of GEICAM 9906 Trial. Breast Cancer Research and Treatment 2005;94(Suppl 1):39.
-
- Rodriguez‐Lescure A, Martin M, Ruiz A, Alba E, Calvo M, Ruiz‐Borrego M. Multicenter, randomized phase 3 study of adjuvant chemotherapy for axillary positive breast cancer (APBC) comparing 6 cycles of FEC vs 4 cycles of FEC followed by 8 weekly paclitaxel administrations: safety analysis of GEICAM 9906 trial. Journal of Clinical Oncology 2004;22(Suppl 14):596.
GOIM 9902 {published data only}
-
- Participating Institutions of GOIM 9902 Trial, Italian Cooperative Group, Rome, Italy. Epirubicin and cyclophosphamide (EC) vs docetaxel followed by EC in adjuvant treatment of node positive breast cancer. A multicenter randomized phase 3 study. Journal of Clinical Oncology 2001;20:1836.
-
- Vici P, Brandi M, Giotta F, Foggi P, Schittulli F, lauro L, et al. A multicenter phase III prospective randomized trial of high‐dose epirubicin in combination with cyclophosphamide (EC) versus docetaxel followed by EC in node‐positive breast cancer. GOIM (Gruppo Oncologico Italia Meridionale) 9902 study. Annals of Oncology 2012;23(5):1121‐9. - PMC - PubMed
GONO MIG‐5 {published data only}
-
- Mastro L, Costantini M, Durando A, Michelotti A, Danese S, Aitini E, et al. Cyclophosphamide, epirubicin, and 5‐fluorouracil versus epirubicin plus paclitaxel in node‐positive early breast cancer patients: a randomized, phase III study of Gruppo Oncologico Nord Ovest‐Mammella Intergruppo Group. Journal of Clinical Oncology 2008;26(Suppl 10):516.
-
- Mastro L, Levaggi A, Michelotti A, Cavazzini G, Adami F, Scotto T, et al. 5‐fluorouracil, epirubicin and cyclophosphamide versus epirubicin and paclitaxel in node‐positive early breast cancer: a phase III randomized GONO‐MIG5 trial. Breast Cancer Research and Treatment 2016;155(1):117‐26. - PubMed
-
- NCT02450058. Adjuvant FEC versus EP in breast cancer (MIG5). clinicaltrials.gov/ct2/show/NCT02450058 (first received 21 May 2015).
-
- Participating Institutions to GONO‐MIG 5 study. Absence of clinically relevant cardiotoxicity in early breast cancer patients treated with the association of epirubicin plus paclitaxel: results from the Italian MIG 5 Study. Journal of Clinical Oncology 2000;19:363.
HeCOG {published data only}
-
- Fountzilas G, Skarlos D, Dafni U, Gogas H, Briasoulis E, Pectasides D, et al. Postoperative dose‐dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high risk operable breast cancer: a randomized phase 3 study conducted by the Hellenic Cooperative Oncology Group. Annals of Oncology 2005;16(11):1762‐71. - PubMed
HORG {published data only}
-
- Polyzos A, Malamos N, Boukovinas I, Adamou A, Ziras N, Kalbakis K, et al. FEC versus sequential docetaxel followed by epirubicin/cyclophosphamide as adjuvant chemotherapy in women with axillary node‐positive early breast cancer: a randomized study of the Hellenic Oncology Research Group (HORG). Breast Cancer Research and Treatment 2010;119(1):95‐104. - PubMed
ICE II‐GBG 52 {published data only}
-
- NCT01204437. Adjuvant chemotherapy for elderly non frail patients with an increased risk for relapse of a primary carcinoma of the breast (ICE‐II). clinicaltrials.gov/ct2/show/NCT01204437 (first received 17 September 2010).
-
- Minckwitz G, Conrad B, Reimer T, Decker T, Eidtmann H, Eiermann W, et al. A randomized phase 2 study comparing EC or CMF versus nab‐paclitaxel plus capecitabine as adjuvant chemotherapy for nonfrail elderly patients with moderate to high‐risk early breast cancer (ICE II‐GBG 52). Cancer 2015;121(20):3639‐48. - PubMed
NCIC‐CTG MA21a {published data only}
-
- Burnell M, Levine M, Chapman JA, et al. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of an interim analysis. Breast Cancer Research and Treatment, San Antonio Breast Cancer Symposium. 2006:53.
-
- Burnell M, Levine MN, Chapman JAW, Bramwell V, Gelmon K, Walley B, et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose‐dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node‐positive or high‐risk node‐negative breast cancer. Journal of Clinical Oncology 2010;28(1):77‐82. - PMC - PubMed
-
- Burnell MJ, Shepherd L, Gelmon K, Bramwell V, Walley B, Vandenberg E, et al. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of the final relapse free survival analysis. Cancer Research 2012;72(Suppl 24):P1‐13‐01.
-
- Participating Organisations. Phase 3 randomized study of adjuvant cyclophosphamide, epirubucin, and fluorouracil versus cyclophosphamide, epirubicin, filgrastim (G‐CSF), and epoetin alfa followed by paclitaxel versus cyclophosphamide and doxorubicin followed by paclitaxel in premenopausal or early postmenopausal women with previously resected node positive or high‐risk node negative stage 1‐3A breast cancer. Protocol only 2001.
NCIC‐CTG MA21b {published data only}
-
- Burnell M, Levine M, Chapman JA, et al. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of an interim analysis. Breast Cancer Research and Treatment, San Antonio Breast Cancer Symposium. 2006:53.
-
- Burnell M, Levine MN, Chapman JAW, Bramwell V, Gelmon K, Walley B, et al. Cyclophosphamide, epirubicin, and fluorouracil versus dose‐dense epirubicin and cyclophosphamide followed by paclitaxel versus doxorubicin and cyclophosphamide followed by paclitaxel in node‐positive or high‐risk node‐negative breast cancer. Journal of Clinical Oncology 2010;28(1):77‐82. - PMC - PubMed
-
- Burnell MJ, Shepherd L, Gelmon K, Bramwell V, Walley B, Vandenberg E, et al. A randomized trial of CEF versus dose dense EC followed by paclitaxel versus AC followed by paclitaxel in women with node positive or high risk node negative breast cancer, NCIC CTG MA.21: results of the final relapse free survival analysis. Cancer Research 2012;72(Suppl 24):P1‐13‐01.
-
- Participating Organisations. Phase 3 randomized study of adjuvant cyclophosphamide, epirubucin, and fluorouracil versus cyclophosphamide, epirubicin, filgrastim (G‐CSF), and epoetin alfa followed by paclitaxel versus cyclophosphamide and doxorubicin followed by paclitaxel in premenopausal or early postmenopausal women with previously resected node positive or high‐risk node negative stage 1‐3A breast cancer. Protocol only issue 2001.
NSABP B‐28 {published data only}
-
- Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node‐positive breast cancer: results from NSABP B‐28. Journal of Clinical Oncology 2005;23(16):3686‐96. - PubMed
PACS 01 {published data only}
-
- Coudert B, Asselain B, Campone M, Spielmann M, Machiels JP, Penault‐Llorca F, et al. Extended benefit from sequential administration of docetaxel after standard fluorouracil, epirubicin, and cyclophosphamide regimen for node‐positive breast cancer: the 8‐year follow‐up results of the UNICANCER‐PACS01 trial. Oncologist 2012;17(7):900‐9. - PMC - PubMed
-
- Marino P, Siani C, Roché H, Protière C, Fumoleau P, Spielmann M, et al. Cost‐effectiveness of adjuvant docetaxel for node‐positive breast cancer patients: results of the PACS 01 economic study. Annals of Oncology 2010;21(7):1448‐54. - PubMed
-
- Roche H, Fumoleau P, Spielmann M, et al. Five years analysis of the PACS 01 Trial: 6 cycles of FEC100 vs 3 cycles of FEC100 followed by 3 cycles of docetaxel for the adjuvant treatment of node positive breast cancer. San Antonio Breast Cancer Symposium. 2004.
-
- Roché H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, et al. Sequential adjuvant epirubicin‐based and docetaxel chemotherapy for node‐positive breast cancer patients: the FNCLCC PACS 01 Trial. Journal of Clinical Oncology 2006;24(36):5664‐71. - PubMed
RAPP‐01 {published data only}
-
- Brain E, Debled M, Eymard J, Bachelot T, Extra J, Serin D, et al. Final results of the RAPP‐01 phase III trial comparing doxorubicin and docetaxel with doxorubicin and cyclophosphamide in the adjuvant treatment of high‐risk node negative and limited node positive (≤ 3) breast cancer patients. Cancer Research 2009;69(2 Suppl 1):4101.
-
- Brain EG, Bachelot T, Serin D, Kirscher S, Graic Y, Eymard J, et al. Life‐threatening sepsis associated with adjuvant doxorubicin plus docetaxel for intermediate‐risk breast cancer. Journal of the American Medical Asssociation 2005;293(19):2367‐71. - PubMed
-
- Brain EGC, Bachelot T, Serin D, Graic Y, Eymard JC, Extra JM, et al. Phase III trial comparing doxorubicin docetaxel (AT) with doxorubicin cyclophosphamide (AC) in the adjuvant treatment of high‐risk node negative (pN0) and limited node positive (pN+</=3) breast cancer (BC) patients (pts): first analysis of toxicity. Journal of Clinical Oncology 2004;22(Suppl 14):617.
Roy {published data only}
-
- Roy C, Choudhury KB, Pal M, Saha A, Bag S, Banerjee C. Adjuvant chemotherapy with six cycles of AC regimen versus three cycles of AC regimen followed by three cycles of paclitaxel in node‐positive breast cancer. Indian Journal of Cancer 2012;49(3):266‐71. - PubMed
Sakr {published data only}
-
- Sakr H, Hamed RH, Anter AH, Yossef T. Sequential docetaxel as adjuvant chemotherapy for node‐positive or/and T3 or T4 breast cancer: clinical outcome (Mansoura University). Medical Oncology 2013;30(1):457. - PubMed
Taxit 216 {published data only}
-
- Bianco AR, Matteis A, Manzione L, Boni C, Palazzo S, Palma M, et al. Sequential epirubicin‐docetaxel‐CMF as adjuvant therapy of early breast cancer: results of the Taxit216 multicenter phase III trial. Journal of Clinical Oncology 2006;24:LBA520.
-
- Cognetti F, Laurentiis M, Matteis A, Manzione L, Boni C, Palazzo S, et al. Sequential epirubicin‐docetaxel‐CMF as adjuvant therapy for node‐positive early stage breast cancer: updated results of the taxit216 randomized trial. Annals of Oncology 2008;19(Suppl 8):viii77–viii88: 1820.
-
- Forestieri V. Docetaxel in adjuvant therapy of breast cancer: results of the TAXIT 216 multicenter phase III trial. Docetaxel in Adjuvant Therapy of Breast Cancer: Results of the TAXIT 216 Multicenter Phase III Trial. Naples, Italy: University of Naples Federico II, 2008.
TITAN {published data only}
-
- NCT00789581. A randomized trial of Ixempra versus Taxol in adjuvant therapy of triple negative breast cancer (TITAN). clinicaltrials.gov/ct2/show/NCT00789581 (first received 13 November 2008).
-
- Yardley DA, Arrowsmith ER, Daniel BR, Eakle J, Brufsky A, Drosick DR, et al. TITAN: phase III study of doxorubicin/cyclophosphamide followed by ixabepilone or paclitaxel in early‐stage triple‐negative breast cancer. Breast Cancer Research and Treatment 2017;164(3):649‐58. - PubMed
-
- Yardley DA, Hainsworth JD, Harwin WN, Goble SA, Daniel BR, Ackerman MA, et al. TITAN: ixabepilone versus weekly paclitaxel following doxorubicin/cyclophosphamide (AC) adjuvant chemotherapy in triple‐negative breast cancer (TNBC): preliminary toxicity of a Sarah Cannon Research Institute phase III trial. Journal of Clinical Oncology. 2011; Vol. 29:1103.
UK TACT {published data only}
-
- Bliss JM, Ellis P, Kilburn L, Bartlett J, Bloomfield D, Cameron D, et al. Mature analysis of UK Taxotere as Adjuvant Chemotherapy (TACT) trial (CRUK 01/001); effects of treatment and characterisation of patterns of breast cancer relapse. Cancer Research 2012;72:P1‐13‐03.
-
- Hopwood P, Ellis P, Barrett‐Lee P, Bliss J, Hall E, Johnson L, et al. Impact of quality of life (QL) during chemotherapy (CT) of FEC‐T compared to FEC or E‐CMF: results from the UK NCRI Taxotere as Adjuvant Chemotherapy trial (TACT). Journal of Clinical Oncology 2005;23(Suppl 16):661.
-
- Participating Organizations. Phase 3 randomized adjuvant study of fluorouracil, epirubicin, and cyclophosphamide (FEC) or epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil versus FEC followed by sequential docetaxel in women with resected stage 1 or 2 breast cancer. Protocol only 2002.
US Oncology 9735 {published data only}
-
- Jones S, Holmes FA, O'Shaughnessy J, Blum JL, Vukelja SJ, Mclntyre KJ, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7‐year follow‐up of US oncology research trial 9735. Journal of Clinical Oncology 2009;27(8):1177‐83. - PubMed
-
- Jones SE, Savin M, Holmes FA, et al. Preliminary results of a prospective randomized trial of adjuvant chemotherapy for patients with stage 1‐3 operable, invasive breast cancer comparing 4 cycles of AC to 4 courses of TC. Journal of Clinical Oncology 2001;20:128.
-
- Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of Clinical Oncology 2006;24(34):5381‐7. - PubMed
References to studies excluded from this review
Albert {published data only}
-
- Albert JM, Buzdar AU, Guzman R, Allen PK, Strom EA, Perkins GH, et al. Prospective randomized trial of 5‐fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus paclitaxel and FAC (TFAC) in patients with operable breast cancer: impact of taxane chemotherapy on locoregional control. Breast Cancer Research and Treatment 2011;128(2):421‐7. - PubMed
Dang {published data only}
-
- Dang C. Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by paclitaxel for early breast cancer. Current Breast Cancer Reports 2009;1(1):1‐2. - PubMed
Di Leo {published data only}
-
- Leo A, Crown J, Nogaret JM, Duffy K, Bartholomeus S, Dolci S, et al. Feasibility of docetaxel‐containing regimens in the adjuvant treatment of breast cancer. Annals of Oncology 2000;11(2):169‐75. - PubMed
Dunphy {published data only}
-
- Dunphy F, Rodriguez J, Petruska P, Velasquez W, McIntyre W, Spitzer G. High dose therapy for high risk (stage 3) breast cancer. Phase II trials of two treatment regimens cytoxan‐etoposide‐cisplatin and cytoxan‐etoposide‐cisplatin‐taxol‐carboplatin. Breast Cancer Research and Treatment 1997;46(1):308.
Hugh {published data only}
Kummel {published data only}
MD Anderson CC {published data only}
-
- Buzdar AU, Singletary SE, Valero V, Booser DJ, Ibrahim NK, Rahman Z, et al. Evaluation of paclitaxel in adjuvant chemotherapy for patients with operable breast cancer: preliminary data of a prospective randomized trial. Clinical Cancer Research 2002;8(5):1073‐9. - PubMed
NCT02838225 {published data only}
-
- NCT02838225. DA versus DAC as postoperative adjuvant treatment for early‐stage breast cancer. clinicaltrials.gov/ct2/show/NCT02838225 (first received 20 July 2016).
NSABP B‐27 {published data only}
-
- Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B‐27. Journal of Clinical Oncology 2006;24(13):2019‐27. - PubMed
Sparano 2015 {published data only}
SWOG S0221 {published data only}
SWOG S9623 {published data only}
-
- Moore H, Green S, Gralow J, Bearman S, Lew D, Barlow W, et al. Intensive dose‐dense compared with high‐dose adjuvant chemotherapy for high‐risk operable breast cancer: Southwest Oncology Group/Intergroup Study 9623. Journal of Clinical Oncology 2007;25(13):1677‐82. - PubMed
-
- Mortimer JE. A comparison of intensive sequential chemotherapy using doxorubicin plus paclitaxel plus cyclophosphamide with high dose chemotherapy and autologous hematopoietic progenitor cell support for primary breast cancer in women with 4‐9 involved axillary lymph nodes, phase 3, Intergroup. Protocol only 2001. [CALGB 9960]
Wildiers {published data only}
-
- Wildiers H. A randomized phase II trial exploring feasibility of densification and optimal sequencing of postoperative adjuvant fluorouracil, epirubicin plus cyclophosphamide (FEC) and docetaxel chemotherapy in patients with high risk primary operable breast cancer. Physician Data Query (PDQ) 2006.
References to studies awaiting assessment
EC‐DOC {published data only}
-
- Gluz O, Erber R, Kates R, Kreipe H, Liedtke C, Pelz E, et al. Predictive value of HER2, topoisomerase‐II (Topo‐II) and tissue inhibitor of metalloproteinases (TIMP‐1) for efficacy of taxane‐based chemotherapy in intermediate risk breast cancer ‐ results of the EC‐Doc Trial. Cancer Research 2011;71:P1‐06‐03. - PubMed
-
- Nitz U, Huober J, Lisboa B, Harbeck N, Fischer H, Moebus V, et al. Interim results of Intergroup EC‐Doc Trial: a randomized multicentre phase III trial comparing adjuvant CEF/CMF to EC‐Docetaxel in patients with 1‐3 positive lymph nodes. Journal of Clinical Oncology 2008;26(Suppl 15):515.
-
- Nitz U, Huober J, Lisboa B, Harbeck N, Fischer H, Moebus V, et al. Superiority of sequential docetaxel over standard FE100C in patients with intermediate risk breast cancer: survival results of the randomized intergroup phase III trial EC‐Doc. Cancer Research 2008;69:78.
EORTC 10041/BIG 3‐04 MINDACT {published data only}
-
- Cardoso F, Piccart‐Gebhart MJ, Rutgers EJ, Litiere S, Van't VL, Viale G, et al. Standard anthracycline‐based vs docetaxel‐capecitabine in early breast cancer: results from the chemotherapy randomization (R‐C) of EORTC 10041/BIG3‐04 MINDACT phase III trial. Journal of Clinical Oncology; 2017 Annual meeting of the American Society of Clinical Oncology. 2017; Vol. 35:15 Suppl 1.
Kader {published data only}
PACS 04 {published data only}
-
- Participating Organizations. Phase 3 randomized study of adjuvant docetaxel and epirubicin versus adjuvant cyclophosphamide, epirubucin, and fluorouracil with or without trastuzumab in women with nonmetastatic adenocarcinoma of the breast with lymph node invasion. Protocol only 2003.
-
- Roché H, Allouache D, Romieu G, Bourgeois H, Canon J, Serin D, et al. Five‐year analysis of the FNCLCC‐PACS04 Trial: FEC100 vs ED75 for the adjuvant treatment of node positive breast cancer. Cancer Research 2009;69:602.
-
- Spielmann M, Roche H, Delozier T, Canon JL, Romieu G, Bourgeois H, et al. Trastuzumab for patients with axillary‐node‐positive breast cancer: results of the FNCLCC‐PACS 04 trial. Journal of Clinical Oncology 2009;27(36):6129‐34. - PubMed
References to ongoing studies
NCI‐H99‐0038 {published data only}
-
- Participating Organizations. Phase 2 randomized study of doxorubicin, cyclophosphamide, and paclitaxel vs cyclophosphamide, thiotepa, and carboplatin in patients with high‐risk primary breast cancer. Protocol only 2001.
NCT01966471 {published data only}
-
- NCT01966471. A study of Kadcyla (trastuzumab emtansine) plus Perjeta (pertuzumab) following anthracyclines in comparison with Herceptin (trastuzumab) plus Perjeta and a taxane following anthracyclines as adjuvant therapy in patients with operable HER2‐positive primary breast cancer. clinicaltrials.gov/show/NCT01966471 (first received 21 October 2013).
NCT02549677 {published data only}
-
- NCT02549677. Epirubicin versus docetaxel plus cyclophosphamide in lymph node negative, ER‐positive, Her2‐negative breast cancer (ELEGANT). clinicaltrials.gov/ct2/show/NCT02549677 (first received 15 September 2015).
NNBC3 {published data only}
-
- Kantelhardt E, Vetter M, Schmidt M, Veyret C, Augustin D, Hanf V, et al. Prospective evaluation of prognostic factors uPA/PAI‐1 in node‐negative breast cancer: phase III NNBC3‐Europe trial (AGO, GBG, EORTC‐PBG) comparing 6 x FEC versus 3 x FEC/3 x docetaxel. BioMed Central 2011;11:140. - PMC - PubMed
Additional references
AIHW 2012
-
- Breast cancer in Australia: an overview. Australian Institute of Health and Welfare & Cancer Australia. Case series no. 71. Canberra: Cat no CAN 67, October 2012.
Bishop 1999
-
- Bishop JF, Dewar J, Toner GC, Smith J, Tattersall MH, Olver IN, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front‐line therapy in untreated metastatic breast cancer. Journal of Clinical Oncology 1999;17(8):2355‐64. - PubMed
Bria 2006
-
- Bria E, Nistico C, Cuppone F, Carlini P, Ciccarese M, Milella M, et al. Benefit of taxanes as adjuvant chemotherapy for early breast cancer: pooled analysis of 15,500 patients. Cancer 2006;106(11):2337‐44. - PubMed
Chan 1999
-
- Chan S, Friedrichs K, Noel D, Pinter T, Belle S, Vorobiof D, et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. The 303 Study Group. Journal of Clinical Oncology 1999;17(8):2341‐54. [MEDLINE: ] - PubMed
Crown 2002
-
- Crown J, Dieras V, Kaufmann M, Minckwitz G, Kaye S, Leonard R, et al. Chemotherapy for metastatic breast cancer ‐ report of a European expert panel. Lancet Oncology 2002;3(12):719‐27. [MEDLINE: ] - PubMed
EBCTCG 2005
-
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15‐year survival: an overview of the randomised trials.. Lancet 2005;365:1687‐1717. - PubMed
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136:E359‐86. - PubMed
Ghersi 2005
GRADEproGDT
-
- GRADEproGDT: GRADEpro Guideline Development Tool [software]. McMaster University, 2015 (developed by Evidence Prime, Inc). Available from www.gradepro.org.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Joensuu 2006
-
- Joensuu H, Kellokumpu‐Lehtinen P, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. New England Journal of Medicine 2006;354:809‐20. - PubMed
NCCN 2007
-
- National Comprehensive Cancer Network Practice Guidelines in Oncology: Breast Cancer; Version 2, 2007. www.nccn.org/professionals/physician_gls/PDF/breast.pdf (accessed 30 April 2007).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [MEDLINE: ] - PubMed
Qin 2011
RevMan [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Tierney 2007
Yusuf 1991
-
- Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991;266(1):93‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

