4D cardiac electromechanical activation imaging
- PMID: 31476587
- PMCID: PMC6817394
- DOI: 10.1016/j.compbiomed.2019.103382
4D cardiac electromechanical activation imaging
Abstract
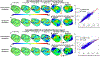
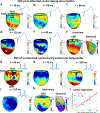
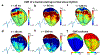
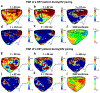
Cardiac abnormalities, a major cause of morbidity and mortality, affect millions of people worldwide. Despite the urgent clinical need for early diagnosis, there is currently no noninvasive technique that can infer to the electrical function of the whole heart in 3D and thereby localize abnormalities at the point of care. Here we present a new method for noninvasive 4D mapping of the cardiac electromechanical activity in a single heartbeat for heart disease characterization such as arrhythmia and infarction. Our novel technique captures the 3D activation wave of the heart in vivo using high volume-rate (500 volumes per second) ultrasound with a 32 × 32 matrix array. Electromechanical activation maps are first presented in a normal and infarcted cardiac model in silico and in canine heart during pacing and re-entrant ventricular tachycardia in vivo. Noninvasive 4D electromechanical activation mapping in a healthy volunteer and a heart failure patient are also determined. The technique described herein allows for direct, simultaneous and noninvasive visualization of electromechanical activation in 3D, which provides complementary information on myocardial viability and/or abnormality to clinical imaging.
Keywords: 3D activation mapping; 3D ultrasound; Cardiac activation mapping; Cardiac arrhythmia; Electromechanical wave imaging; High frame rate ultrasound.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Electromechanical wave imaging (EWI) validation in all four cardiac chambers with 3D electroanatomic mapping in canines in vivo.Phys Med Biol. 2016 Nov 21;61(22):8105-8119. doi: 10.1088/0031-9155/61/22/8105. Epub 2016 Oct 26. Phys Med Biol. 2016. PMID: 27782003 Free PMC article.
-
Validation of electromechanical wave imaging in a canine model during pacing and sinus rhythm.Heart Rhythm. 2016 Nov;13(11):2221-2227. doi: 10.1016/j.hrthm.2016.08.010. Epub 2016 Aug 4. Heart Rhythm. 2016. PMID: 27498277 Free PMC article.
-
Insights from Novel Noninvasive CT and ECG Imaging Modalities on Electromechanical Myocardial Activation in a Canine Model of Ischemic Dyssynchronous Heart Failure.J Cardiovasc Electrophysiol. 2016 Dec;27(12):1454-1461. doi: 10.1111/jce.13091. Epub 2016 Oct 13. J Cardiovasc Electrophysiol. 2016. PMID: 27578532
-
Imaging the Propagation of the Electromechanical Wave in Heart Failure Patients with Cardiac Resynchronization Therapy.Pacing Clin Electrophysiol. 2017 Jan;40(1):35-45. doi: 10.1111/pace.12964. Epub 2016 Dec 2. Pacing Clin Electrophysiol. 2017. PMID: 27790723 Free PMC article. Review.
-
Evolution of mapping and anatomic imaging of cardiac arrhythmias.Pacing Clin Electrophysiol. 2004 Jul;27(7):1026-49. doi: 10.1111/j.1540-8159.2004.00581.x. Pacing Clin Electrophysiol. 2004. PMID: 15271032 Review. No abstract available.
Cited by
-
Electromechanical Cycle Length Mapping for atrial arrhythmia detection and cardioversion success assessment.Comput Biol Med. 2023 Sep;163:107084. doi: 10.1016/j.compbiomed.2023.107084. Epub 2023 May 30. Comput Biol Med. 2023. PMID: 37302374 Free PMC article.
-
Electrical Impedance Tomography - Recent Applications and Developments.J Electr Bioimpedance. 2021 Nov 20;12(1):50-62. doi: 10.2478/joeb-2021-0007. eCollection 2021 Jan. J Electr Bioimpedance. 2021. PMID: 35069942 Free PMC article.
-
Reconstruction of excitation waves from mechanical deformation using physics-informed neural networks.Sci Rep. 2024 Jul 23;14(1):16975. doi: 10.1038/s41598-024-67597-3. Sci Rep. 2024. PMID: 39043805 Free PMC article.
-
Noninvasive localization of cardiac arrhythmias using electromechanical wave imaging.Sci Transl Med. 2020 Mar 25;12(536):eaax6111. doi: 10.1126/scitranslmed.aax6111. Sci Transl Med. 2020. PMID: 32213631 Free PMC article.
-
Cardiac Resynchronization Therapy Response Assessment with Electromechanical Activation Mapping within 24 Hours of Device Implantation: A Pilot Study.J Am Soc Echocardiogr. 2021 Jul;34(7):757-766.e8. doi: 10.1016/j.echo.2021.02.012. Epub 2021 Mar 4. J Am Soc Echocardiogr. 2021. PMID: 33675941 Free PMC article.
References
-
- Lin HJ et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 27, 1760–1764 (1996). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

