Pim1 Impacts Enterovirus A71 Replication and Represents a Potential Target in Antiviral Therapy
- PMID: 31476618
- PMCID: PMC6726883
- DOI: 10.1016/j.isci.2019.08.008
Pim1 Impacts Enterovirus A71 Replication and Represents a Potential Target in Antiviral Therapy
Abstract
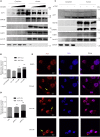
Enterovirus A71 (EV-A71) infection causes hand-foot-and-mouth disease (HFMD) and fatal neurological diseases, and there are no effective treatments. Host factors play key roles in establishing viral infection and determining the disease progression and outcome of antiviral therapies. In this study, we found that the expression of Pim1 was significantly upregulated in EV-A71 infection. Ectopic expression or silencing of Pim1 promoted or inhibited EV-A71 replication through two distinct mechanisms. Pim1 enhanced viral IRES activity by increasing viral 2A protease-mediated eIF4G cleavage and blocked AUF1, a suppressor of IRES, translocation from the nucleus to cytosol. More importantly, we discovered that Pim1 inhibitors (SGI-1776, AZD-1208, and CX-6258) reduced EV-A71 reproduction. Particularly, CX-6258 remarkably reduced EV-A71 reproduction more than 1,000 times, providing a potential therapeutic agent for EV-A71 treatment.
Keywords: Biochemistry; Biological Sciences; Cell Biology; Microbiology; Molecular Microbiology; Viral Microbiology; Virology.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Hsp27 Responds to and Facilitates Enterovirus A71 Replication by Enhancing Viral Internal Ribosome Entry Site-Mediated Translation.J Virol. 2019 Apr 17;93(9):e02322-18. doi: 10.1128/JVI.02322-18. Print 2019 May 1. J Virol. 2019. PMID: 30814282 Free PMC article.
-
Hsc70 regulates the IRES activity and serves as an antiviral target of enterovirus A71 infection.Antiviral Res. 2018 Feb;150:39-46. doi: 10.1016/j.antiviral.2017.11.020. Epub 2017 Nov 24. Antiviral Res. 2018. PMID: 29180285
-
Translocating lipopolysaccharide correlates with the severity of enterovirus A71-induced HFMD by promoting pro-inflammation and viral IRES activity.Gut Pathog. 2021 Nov 22;13(1):69. doi: 10.1186/s13099-021-00465-x. Gut Pathog. 2021. PMID: 34809671 Free PMC article.
-
Interplays between Enterovirus A71 and the innate immune system.J Biomed Sci. 2019 Dec 2;26(1):95. doi: 10.1186/s12929-019-0596-8. J Biomed Sci. 2019. PMID: 31787104 Free PMC article. Review.
-
[Progress in Research on Structure, Function and Antiviral of Enterovirus A71 3C Protein].Bing Du Xue Bao. 2015 Jul;31(4):468-73. Bing Du Xue Bao. 2015. PMID: 26524922 Review. Chinese.
Cited by
-
IRF2 Cooperates with Phosphoprotein of Spring Viremia of Carp Virus to Suppress Antiviral Response in Zebrafish.J Virol. 2022 Nov 23;96(22):e0131422. doi: 10.1128/jvi.01314-22. Epub 2022 Oct 31. J Virol. 2022. PMID: 36314827 Free PMC article.
-
Suppressing MDSC Infiltration in Tumor Microenvironment Serves as an Option for Treating Ovarian Cancer Metastasis.Int J Biol Sci. 2022 May 21;18(9):3697-3713. doi: 10.7150/ijbs.70013. eCollection 2022. Int J Biol Sci. 2022. PMID: 35813475 Free PMC article.
-
Targeting m6A modification inhibits herpes virus 1 infection.Genes Dis. 2021 Feb 22;9(4):1114-1128. doi: 10.1016/j.gendis.2021.02.004. eCollection 2022 Jul. Genes Dis. 2021. PMID: 35685469 Free PMC article.
-
Targeting WDPF domain of Hsp27 achieves a broad spectrum of antiviral.MedComm (2020). 2025 Feb 26;6(3):e70032. doi: 10.1002/mco2.70032. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 40013315 Free PMC article.
-
Pim1 promotes IFN-β production by interacting with IRF3.Exp Mol Med. 2022 Nov;54(11):2092-2103. doi: 10.1038/s12276-022-00893-y. Epub 2022 Nov 29. Exp Mol Med. 2022. PMID: 36446848 Free PMC article.
References
-
- De Vries M., Smithers N.P., Howarth P.H., Nawijn M.C., Davies D.E. Inhibition of Pim1 kinase reduces viral replication in primary bronchial epithelial cells. Eur. Respir. J. 2015;45:1745–1748. - PubMed
-
- Dong Q., Men R., Dan X., Chen Y., Li H., Chen G., Zee B., Wang M.H.T., He M.L. Hsc70 regulates the IRES activity and serves as an antiviral target of enterovirus A71 infection. Antiviral. Res. 2018;150:39–46. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

