Fetal biometry for guiding the medical management of women with gestational diabetes mellitus for improving maternal and perinatal health
- PMID: 31476798
- PMCID: PMC6718273
- DOI: 10.1002/14651858.CD012544.pub2
Fetal biometry for guiding the medical management of women with gestational diabetes mellitus for improving maternal and perinatal health
Abstract
Background: Gestational diabetes mellitus (GDM) is a common medical condition that complicates pregnancy and causes adverse maternal and fetal outcomes. At present, most treatment strategies focus on normalisation of maternal blood glucose values with use of diet, lifestyle modification, exercise, oral anti-hyperglycaemics and insulin. This has been shown to reduce the incidence of adverse outcomes, such as birth trauma and macrosomia. However, this involves intensive monitoring and treatment of all women with GDM. We propose that using medical imaging to identify pregnancies displaying signs of being affected by GDM could help to target management, allowing low-risk women to be spared excessive intervention, and facilitating better resource allocation.
Objectives: We wanted to address the following question: in women with gestational diabetes, does the use of fetal imaging plus maternal blood glucose concentration to indicate the need for medical management compared with glucose concentration alone reduce the risk of adverse perinatal outcomes?
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register (29 January 2019), ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP) (both on 29 January 2019), and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials, including those published in abstract form only. Studies using a cluster-randomised design and quasi-randomised controlled trials were both eligible for inclusion, but we didn't identify any. Cross-over trials were not eligible for inclusion in our review.We included women carrying singleton pregnancies who were diagnosed with GDM, as defined by the trials' authors. The intervention of interest was the use of fetal biometry on imaging methods in addition to maternal glycaemic values for indicating the use of medical therapy for GDM. The control group was the use of maternal glycaemic values alone for indicating the use of such therapy.
Data collection and analysis: Two review authors independently assessed trials for inclusion and assessed risk of bias. Two review authors extracted data and checked them for accuracy.
Main results: Three randomised controlled trials met the inclusion criteria for our systematic review - the studies randomised a total of 524 women.We assessed the three included studies as being at a low to moderate risk of bias; the nature of the intervention made it difficult to achieve blinding of participants and personnel and none of the trial reports contained information about methods of allocation concealment (and were therefore assessed as being at an unclear risk of selection bias).In all studies, the intervention was the use of fetal biometry on ultrasound to identify fetuses displaying signs of fetal macrosomia, and the use of this information to indicate the use of medical anti-hyperglycaemic treatments. Those pregnancies were subject to more stringent blood glucose targets than those without signs of fetal macrosomia.Maternal outcomesThe use of fetal biometry in addition to maternal blood glucose concentration (compared with maternal blood glucose concentration alone) may make little or no difference to the incidence of caesarean delivery (risk ratio (RR) 0.81, 95% confidence interval (CI) 0.59 to 1.10; 2 trials, 428 women; low-certainty evidence). We are unclear about the results for hypertensive disorders of pregnancy (RR 0.80, 95% CI 0.34 to 1.89; 2 trials, 325 women) due to very low-certainty evidence. The included trials did not report on development of type 2 diabetes in the mother or maternal hypoglycaemia.Fetal and neonatal outcomesThe use of fetal biometry may make little or no difference to the incidence of neonatal hypoglycaemia (RR 0.90, 95% CI 0.57 to 1.42; 3 trials, 524 women; low-certainty evidence). Very low-certainty evidence means that we are unclear about the results for large-for-gestational age (RR 0.81, 95% CI 0.38 to 1.74; 3 trials, 524 women); shoulder dystocia (RR 0.33, 95% CI 0.01 to 7.98; 1 trial, 96 women); a composite measure of perinatal morbidity or mortality (RR 1.00, 95% CI 0.21 to 4.71; 1 study, 96 women); or perinatal mortality (RR 0.33, 95% CI 0.01 to 7.98; 1 trial, 96 women).
Authors' conclusions: This review is based on evidence from three trials involving 524 women. The trials did not report some important outcomes of interest to this review, and the majority of our secondary outcomes were also unreported. The available evidence ranged from low- to very low-certainty, with downgrading decisions based on limitations in study design, imprecision and inconsistency.There is insufficient evidence to evaluate the use of fetal biometry (in addition to maternal blood glucose concentration values) to assist in guiding the medical management of GDM, on either maternal or perinatal health outcomes, or the associated costs.More research is required, ideally larger randomised studies which report the maternal and infant short- and long-term outcomes listed in this review, as well as those outcomes relating to financial and resource implications.
Conflict of interest statement
Ujvala Rao: none known
Bradley de Vries: none known
Glynis Ross has received payment for lectures from Medtronic (for a lecture to health professionals on insulin pumps and pregnancy) and MSD (a presentation to educators on diabetes and type 2 diabetes; also on diabetes and pregnancy to overseas visiting doctors). None of these lectures/presentations were related to the current review. Glynis Ross has also received payment for the development of educational presentations: Medtronic sponsored presentations on diabetes and pregnancy to GPs, educators, dieticians, specialists in urban and rural areas. Sanofi sponsored the development of educational presentations on diabetes inpatient care at regional hospitals.
Adrienne Gordon: none known
Figures
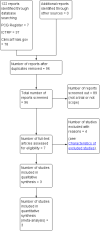

























Update of
References
References to studies included in this review
Bonomo 2004 {published data only}
-
- Bonomo M, Cetin I, Pisoni MP, Faden D, Mion E, Taricco E, et al. Flexible treatment of gestational diabetes modulated on ultrasound evaluation of intrauterine growth: a controlled randomized clinical trial. Diabetes & Metabolism 2004;30(3):237‐44. - PubMed
Kjos 2001 {published data only}
-
- Kjos SL, Schaefer‐Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, et al. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care 2001;24:1904‐10. - PubMed
Schaefer‐Graf 2004 {published data only}
-
- Schaefer‐Graf UM, Kjos SL, Fauzan OH, Buhling KJ, Siebert G, Buhrer C, et al. A randomized trial evaluating a predominately fetal growth‐based strategy to guide management of gestational diabetes in caucasian women. Diabetes Care 2004;27(2):297‐302. - PubMed
References to studies excluded from this review
Buchanan 1994 {published data only}
-
- Buchanan TA, Kjos SL, Montoro MN, Wu PYK, Madrilejo NG, Gonzalez M, et al. Use of fetal ultrasound to select metabolic therapy for pregnancies complicated by mild gestational diabetes. Diabetes Care 1994;17:275‐83. - PubMed
Hopp 1996 {published data only}
-
- Hopp H, Vollert W, Ragosch V, Novak A, Weitzel HK, Glockner E, et al. Indication and results of insulin therapy for gestational diabetes mellitus. Journal of Perinatal Medicine 1996;24:521‐30. - PubMed
Novak 1994 {published data only}
-
- Novak A, Hopp H, Vollert W, Weitzel H, Glockner E. Fetal indication for insulin therapy in gestational diabetes. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994. [Abstract no. 318]
Rossi 2000 {published data only}
-
- Rossi G, Somigliana E, Moschetta M, Bottani B, Barbieri M, Vignali M. Adequate timing of fetal ultrasound to guide metabolic therapy in mild gestational diabetes mellitus. Results from a randomized study. Acta Obstetricia et Gynecologica Scandinavica 2000;79(8):649‐54. - PubMed
Additional references
ADA 2017
-
- American Diabetes Association. Standards of care. Diabetes Care 2017;40(1):114‐9.
AIHW 2014
-
- Australian Institute of Health and Welfare. Australia's Health 2014. www.aihw.gov.au/reports/australias‐health/australias‐health‐2014 (accessed 5 May 2016) 2014.
Black 2013
Coomarasamy 2005
-
- Coomarasamy A, Connock M, Thornton J, Khan KS. Accuracy of ultrasound biometry in the prediction of macrosomia: a systematic quantitative review. BJOG: an international journal of obstetrics and gynaecology 2005;112(11):1461‐6. [PUBMED: 16225563] - PubMed
Crowther 2005
-
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, on behalf of the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352(24):2477‐86. - PubMed
De Reu 2008
-
- Reu PA, Smits LJ, Oosterbaan HP, Nijhuis JG. Value of a single early third trimester fetal biometry for the prediction of birth weight deviations in a low risk population. Journal of Perinatal Medicine 2008;36(4):324‐9. - PubMed
Dudley 2005
-
- Dudley NJ. A systematic review of the ultrasound estimation of fetal weight. Ultrasound in Obstetrics and Gynecology 2005;25(1):80‐9. - PubMed
Duran 2014
-
- Duran A, Sáenz S, Torrejón MJ, Bordiú E, Valle L, Galindo M, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care 2014;37(9):2442‐50. - PubMed
Feig 2015
-
- Feig DS, Corcoy R, Jensen DM, Kautzky‐Willer A, Nolan CJ, Oats JJ, et al. Diabetes in pregnancy outcomes: a systematic review and proposed codification of definitions. Diabetes/metabolism Research and Reviews 2015;31(7):680‐90. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 May 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gull 2002
-
- Gull I, Fait G, Har‐Toov J, Kupferminc MJ, Lessing JB, Jaffa AJ, et al. Prediction of fetal weight by ultrasound: the contribution of additional examiners. Ultrasound in Obstetrics and Gynecology 2002;20:57‐60. - PubMed
Han 2012
HAPO 2008
-
- The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358:1991. - PubMed
Hatab 2008
-
- Hatab MR, Zaretsky MV, Alexander JM, Twickler DM. Comparison of fetal biometric values with sonographic and 3D reconstruction MRI in term gestations. American Journal of Roentgenology 2008;191(2):340‐5. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Karagiannis 2010
Keating 2013
-
- Keating C, Harrison C, Lombard C, Boyle J, Moodie M, Teede H. Healthcare costs associated with gestational diabetes mellitus during pregnancy and potential cost effectiveness in high risk women. Australian and New Zealand Obesity Society Annual Scientific Meeting; 2013 Oct 19; Melbourne, Australia. 2013.
Kolu 2012
Kurmanavicius 2005
-
- Kurmanavicius J, Wright EM, Royston P. Fetal ultrasound biometry: 1: Head reference values. BJOG: an international journal of obstetrics and gynaecology 2005;106:126–5. - PubMed
Liao 2014
-
- Liao S, Mei J, Song W, Liu Y, Tan YD, Chi S, et al. The impact of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) fasting glucose diagnostic criterion on the prevalence and outcomes of gestational diabetes mellitus in Han Chinese women. Diabetic Medicine 2014;31(3):341‐51. - PubMed
Lu 2016
Luesley 2010
-
- Luesley D, Baker P. Chapter 7.5: Gestational diabetes. In: Hodder Education editor(s). Obstetrics and Gynaecology: An Evidence Based Text for MRCOG. London UK: CRC Press, 2010:435‐471.
McGee 2002
Nankervis 2018
-
- Nankervis A, Price S, Conn J. Gestational diabetes mellitus: a pragmatic approach to diagnosis and management. Australian Journal of General Practice 2018;47(7):445‐9. - PubMed
Nelson‐Piercy 2010
-
- Nelson‐Piercy C. Diabetes. Handbook of Obstetric Medicine. 4th Edition. London (UK): Informa Healthcare, 2010:79‐95.
NICE 2015
-
- National Collaborating Centre for Women's and Children's Health. NICE Guideline NG3. Diabetes in Pregnancy: Management of Diiabetes and its Complications from Pre‐Conception to the Postnatal Period. www.nice.org.uk/Guidance/NG3. National Institute for Health and Clinical Excellence, 2015.
Nicholson 2008
Predanic 2002
-
- Predanic M, Cho A, Ingrid F, Pellettieri J. Ultrasonographic estimation of fetal weight: acquiring accuracy in residency. Journal of Ultrasound Medicine 2002;21(5):495‐500. - PubMed
Queensland 2015
-
- Queensland Clinical Guidelines. Gestational Diabetes Mellitus. www.health.qld.gov.au/__data/assets/pdf_file/0023/140099/g‐gdm.pdf. Queensland Clinical Guideline steering committee, Accessed prior to 5 Aug 2019:20‐2.
Rani 2016
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosati 2010
-
- Rosati P, Arduini, M, Giri, C, Guariglia, L. Ultrasonographic weight estimation in large for gestational age fetuses: a comparison of 17 sonographic formulas and four models algorithms. Journal of Maternal Fetal and Neonatal Medicine 2010;23(7):675‐80. - PubMed
Schaefer‐Graf 2003
Schünemann 2013
-
- Schünemann H, Broźek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sovio 2016
-
- Sovio U, Murphy HR, Smith GC. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care 2016;39(6):982‐7. - PubMed
Weiner 1985
-
- Weiner CP, Sabbagha RE, Vaisrub N, Socol ML. Ultrasonic fetal weight prediction: role of head circumference and femur length. Obstetrics and Gynecology 1985;65(6):812‐7. - PubMed
Weiss 1986
-
- Weiss PA, Hofmann HM, Winter RR, Lichtenegger W, Pürstner P, Haas J. Diagnosis and treatment of gestational diabetes according to amniotic fluid insulin levels. Archives of Gynaecology 1986;239(2):81‐91. - PubMed
Wong 2017
-
- Wong V, Lin A, Russell H. Adopting the new World Health Organization diagnostic criteria for gestational diabetes: How the prevalence changes in a high‐risk region in Australia. Diabetes Research and Clinical Practice 2017;129:148‐53. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

