Efficacy of Brentuximab Vedotin in Relapsed or Refractory High-CD30-Expressing Non-Hodgkin Lymphomas: Results of a Multicenter, Open-Labeled Phase II Trial
- PMID: 31476851
- PMCID: PMC7176958
- DOI: 10.4143/crt.2019.198
Efficacy of Brentuximab Vedotin in Relapsed or Refractory High-CD30-Expressing Non-Hodgkin Lymphomas: Results of a Multicenter, Open-Labeled Phase II Trial
Abstract
Purpose: The treatment outcome of brentuximab vedotin (BV) has not been related with CD30 expression in previous studies enrolling patients with a wide range of CD30 expression level. Thus, this study explored the efficacy of BV in high-CD30-expressing non-Hodgkin lymphoma (NHL) patients most likely to benefit.
Materials and methods: This phase II study (Clinicaltrials.gov: NCT02280785) enrolled relapsed or refractory high-CD30-expressing NHL, with BV administered intravenously at 1.8 mg/kg every 3 weeks. The primary endpoint was > 40% disease control rate, consisting of complete response (CR), partial response (PR), or stable disease. We defined high CD30 expression as ≥ 30% tumor cells positive for CD30 by immunohistochemistry.
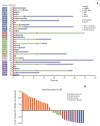
Results: High-CD30-expressing NHL patients (n=33) were enrolled except anaplastic large cell lymphoma. The disease control rate was 48.5% (16/33) including six CR and six PR; six patients (4CR, 2PR) maintained their response over 16 completed cycles. Response to BV and survival were not associated with CD30 expression levels. Over a median of 29.2 months of follow-up, the median progression-free and overall survival rates were 1.9 months and 6.1 months, respectively. The most common adverse events were fever (39%), neutropenia (30%), fatigue (24%), and peripheral sensory neuropathy (27%). In a post-hoc analysis for the association of multiple myeloma oncogene 1 (MUM1) on treatment outcome, MUM1- negative patients showed a higher response (55.6%, 5/9) than MUM1-positive patients (13.3%, 2/15).
Conclusion: BV performance as a single agent was acceptable in terms of disease control rates and toxicity profiles, especially MUM1-negative patients.
Keywords: Brentuximab vedotin; CD30; Multiple myeloma oncogene-1; Non-Hodgkin lymphoma.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures


References
-
- Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–65. - PubMed
-
- Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

