Combinatorial Treatment Using Umbilical Cord Perivascular Cells and Aβ Clearance Rescues Vascular Function Following Transient Hypertension in a Rat Model of Alzheimer Disease
- PMID: 31476904
- PMCID: PMC6739147
- DOI: 10.1161/HYPERTENSIONAHA.119.13187
Combinatorial Treatment Using Umbilical Cord Perivascular Cells and Aβ Clearance Rescues Vascular Function Following Transient Hypertension in a Rat Model of Alzheimer Disease
Abstract
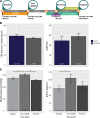
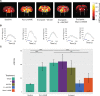
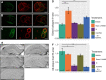
Transient hypertension is a risk factor for Alzheimer disease (AD), but the effects of this interaction on brain vasculature are understudied. Addressing vascular pathology is a promising avenue to potentiate the efficacy of treatments for AD. We used arterial spin labeling magnetic resonance imaging to longitudinally assess brain vascular function and immunohistopathology to examine cerebrovascular remodeling and amyloid load. Hypertension was induced for 1 month by administration of l-NG-nitroarginine-methyl-ester in TgF344-AD rats at the prodromal stage. Following hypertension, nontransgenic rats showed transient cerebrovascular changes, whereas TgF344-AD animals exhibited sustained alterations in cerebrovascular function. Human umbilical cord perivascular cells in combination with scyllo-inositol, an inhibitor of Aβ oligomerization, resulted in normalization of hippocampal vascular function and remodeling, in contrast to either treatment alone. Prodromal stage hypertension exacerbates latter AD pathology, and the combination of human umbilical cord perivascular cells with amyloid clearance promotes cerebrovascular functional recovery.
Keywords: Alzheimer disease; hypertension; inositol; risk factor; umbilical cord.
Figures



Similar articles
-
β-Amyloid, cholinergic transmission, and cerebrovascular system -- a developmental study in a mouse model of Alzheimer's disease.Curr Pharm Des. 2013;19(38):6749-65. doi: 10.2174/13816128113199990711. Curr Pharm Des. 2013. PMID: 23530514
-
Cerebrovascular damage after midlife transient hypertension in non-transgenic and Alzheimer's disease rats.Brain Res. 2021 May 1;1758:147369. doi: 10.1016/j.brainres.2021.147369. Epub 2021 Feb 12. Brain Res. 2021. PMID: 33582120 Free PMC article.
-
Prenatal high-fat diet alters the cerebrovasculature and clearance of β-amyloid in adult offspring.J Pathol. 2015 Mar;235(4):619-31. doi: 10.1002/path.4468. Epub 2015 Jan 7. J Pathol. 2015. PMID: 25345857
-
Pathophysiological Links Among Hypertension and Alzheimer's Disease.High Blood Press Cardiovasc Prev. 2016 Mar;23(1):3-7. doi: 10.1007/s40292-015-0108-1. Epub 2015 Jun 9. High Blood Press Cardiovasc Prev. 2016. PMID: 26054481 Review.
-
The role of perivascular innervation and neurally mediated vasoreactivity in the pathophysiology of Alzheimer's disease.Clin Sci (Lond). 2017 Jun 1;131(12):1207-1214. doi: 10.1042/CS20160769. Clin Sci (Lond). 2017. PMID: 28566449 Review.
Cited by
-
Parvalbumin neuroplasticity compensates for somatostatin impairment, maintaining cognitive function in Alzheimer's disease.Transl Neurodegener. 2022 May 3;11(1):26. doi: 10.1186/s40035-022-00300-6. Transl Neurodegener. 2022. PMID: 35501886 Free PMC article.
-
Blood-brain barrier leakage in Alzheimer's disease: From discovery to clinical relevance.Pharmacol Ther. 2022 Jun;234:108119. doi: 10.1016/j.pharmthera.2022.108119. Epub 2022 Jan 30. Pharmacol Ther. 2022. PMID: 35108575 Free PMC article. Review.
-
TgF344-AD Rat Model of Alzheimer's Disease: Spatial Disorientation and Asymmetry in Hemispheric Neurodegeneration.J Alzheimers Dis Rep. 2023 Sep 26;7(1):1085-1094. doi: 10.3233/ADR-230038. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 37849636 Free PMC article.
-
Insights From TgF344-AD, a Double Transgenic Rat Model in Alzheimer's Disease Research.Physiol Res. 2025 Mar 21;74(1):1-17. doi: 10.33549/physiolres.935464. Physiol Res. 2025. PMID: 40116546 Free PMC article. Review.
-
Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer's disease.Geroscience. 2023 Jun;45(3):1471-1490. doi: 10.1007/s11357-023-00773-x. Epub 2023 Mar 18. Geroscience. 2023. PMID: 36933144 Free PMC article.
References
-
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. - PubMed
-
- Hampel H, Lista S. Alzheimer disease: from inherited to sporadic AD-crossing the biomarker bridge. Nat Rev Neurol. 2012;8:598–600. doi: 10.1038/nrneurol.2012.202. - PubMed
-
- Orgeta V, Mukadam N, Sommerlad A, Livingston G. The lancet commission on dementia prevention, intervention, and care: a call for action. Irish J Psychol Med. 2018;36:85–88. doi: 10.1017/ipm.2018.4. - PubMed
-
- Gorelick PB. Risk factors for vascular dementia and A lzheimer disease. Stroke. 2004;35(11 suppl 1):2620–2622. doi: 10.1161/01.STR.0000143318.70292.47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

