Comparative genomic analysis of six Glossina genomes, vectors of African trypanosomes
- PMID: 31477173
- PMCID: PMC6721284
- DOI: 10.1186/s13059-019-1768-2
Comparative genomic analysis of six Glossina genomes, vectors of African trypanosomes
Abstract
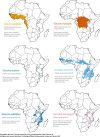
Background: Tsetse flies (Glossina sp.) are the vectors of human and animal trypanosomiasis throughout sub-Saharan Africa. Tsetse flies are distinguished from other Diptera by unique adaptations, including lactation and the birthing of live young (obligate viviparity), a vertebrate blood-specific diet by both sexes, and obligate bacterial symbiosis. This work describes the comparative analysis of six Glossina genomes representing three sub-genera: Morsitans (G. morsitans morsitans, G. pallidipes, G. austeni), Palpalis (G. palpalis, G. fuscipes), and Fusca (G. brevipalpis) which represent different habitats, host preferences, and vectorial capacity.
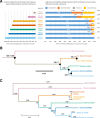
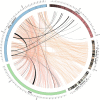
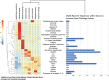
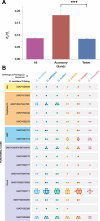
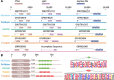
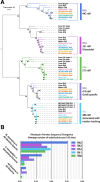
Results: Genomic analyses validate established evolutionary relationships and sub-genera. Syntenic analysis of Glossina relative to Drosophila melanogaster shows reduced structural conservation across the sex-linked X chromosome. Sex-linked scaffolds show increased rates of female-specific gene expression and lower evolutionary rates relative to autosome associated genes. Tsetse-specific genes are enriched in protease, odorant-binding, and helicase activities. Lactation-associated genes are conserved across all Glossina species while male seminal proteins are rapidly evolving. Olfactory and gustatory genes are reduced across the genus relative to other insects. Vision-associated Rhodopsin genes show conservation of motion detection/tracking functions and variance in the Rhodopsin detecting colors in the blue wavelength ranges.
Conclusions: Expanded genomic discoveries reveal the genetics underlying Glossina biology and provide a rich body of knowledge for basic science and disease control. They also provide insight into the evolutionary biology underlying novel adaptations and are relevant to applied aspects of vector control such as trap design and discovery of novel pest and disease control strategies.
Keywords: Disease; Hematophagy; Lactation; Neglected; Symbiosis; Trypanosomiasis; Tsetse.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures





References
-
- Lyons M. The colonial disease. A social history of sleeping sickness in norther Zaire, 1900–1940. Cambridge UK: Cambridge University Press; 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

