Aβ43 in human Alzheimer's disease: effects of active Aβ42 immunization
- PMID: 31477180
- PMCID: PMC6717966
- DOI: 10.1186/s40478-019-0791-6
Aβ43 in human Alzheimer's disease: effects of active Aβ42 immunization
Abstract
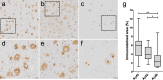
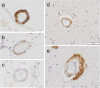
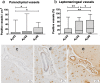
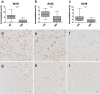
Neuropathological follow-up of patients with Alzheimer's disease (AD) who participated in the first clinical trial of Amyloid-β 42 (Aβ42) immunization (AN1792, Elan Pharmaceuticals) has shown that immunization can induce removal of Aβ42 and Aβ40 from plaques, whereas analysis of the cerebral vessels has shown increased levels of these Aβ peptides in cerebral amyloid angiopathy (CAA). Aβ43 has been less frequently studied in AD, but its aggregation propensity and neurotoxic properties suggest it may have an important pathogenic role. In the current study we show by using immunohistochemistry that in unimmunized AD patients Aβ43 is a frequent constituent of plaques (6.0% immunostained area), similar to Aβ42 (3.9% immunostained area). Aβ43 immunostained area was significantly higher than that of Aβ40 (2.3%, p = 0.006). In addition, we show that Aβ43 is only a minor component of CAA in both parenchymal vessels (1.5 Aβ43-positive vessels per cm2 cortex vs. 5.3 Aβ42-positive vessels, p = 0.03, and 6.2 Aβ40-positive vessels, p = 0.045) and leptomeningeal vessels (5.6% Aβ43-positive vessels vs. 17.3% Aβ42-positive vessels, p = 0.007, and 27.4% Aβ40-positive vessels, p = 0.003). Furthermore, we have shown that Aβ43 is cleared from plaques after Aβ immunotherapy, similar to Aβ42 and Aβ40. Cerebrovascular Aβ43 levels did not change after immunotherapy.
Keywords: Alzheimer’s disease; Amyloid-β; Aβ immunotherapy; Aβ43; Cerebral amyloid angiopathy; Human study; Immunohistochemistry.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
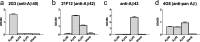



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

