Epigenetic Regulation of Hippocampal Fosb Expression Controls Behavioral Responses to Cocaine
- PMID: 31477569
- PMCID: PMC6794929
- DOI: 10.1523/JNEUROSCI.0800-19.2019
Epigenetic Regulation of Hippocampal Fosb Expression Controls Behavioral Responses to Cocaine
Abstract
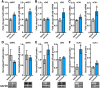
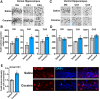
Drug addiction results in part from maladaptive learning, including the formation of strong associations between the drug and the circumstances of consumption. However, drug-induced changes in gene expression underlying the saliency of these associations remain understudied. Consolidation of explicit memories occurs within the hippocampus, and we have shown that spatial learning induces expression of the transcription factor ΔFosB in hippocampus and that this induction is critical for learning. Drugs of abuse also upregulate ΔFosB in hippocampus, but the mechanism of its induction by cocaine and its role in hippocampus-dependent cocaine responses is unknown. We investigated differences in mouse dorsal and ventral hippocampal ΔFosB expression in response to chronic cocaine, because these regions appear to regulate distinct cocaine-related behaviors. We found that cocaine-mediated induction of ΔFosB was subregion-specific, and that ΔFosB transcriptional activity in both the dorsal and ventral hippocampus is necessary for cocaine conditioned place preference. Further, we characterize changes in histone modifications at the FosB promoter in hippocampus in response to chronic cocaine and found that locus-specific epigenetic modification is essential for FosB induction and multiple hippocampus-dependent behaviors, including cocaine place preference. Collectively, these findings suggest that exposure to cocaine induces histone modification at the hippocampal FosB gene promoter to cause ΔFosB induction critical for cocaine-related learning.SIGNIFICANCE STATEMENT Although cocaine addiction is driven in part by the formation of indelible associations between the drug and the environment, paraphernalia, and circumstances of use, and although this type of associative learning is dependent upon changes in gene expression in a brain region called the hippocampus, the mechanisms by which cocaine alters hippocampal gene expression to drive formation of these associations is poorly understood. Here, we demonstrate that chronic cocaine engages locus-specific changes in the epigenetic profile of the FosB gene in the hippocampus, and that these alterations are required for cocaine-dependent gene expression and cocaine-environment associations. This work provides novel insight into addiction etiology and potential inroads for therapeutic intervention in cocaine addiction.
Keywords: cocaine; epigenetics; hippocampus; histone; transcription; ΔFosB.
Copyright © 2019 the authors.
Figures




References
-
- Cates HM, Thibault M, Pfau M, Heller E, Eagle A, Gajewski P, Bagot R, Colangelo C, Abbott T, Rudenko G, Neve R, Nestler EJ, Robison AJ (2014) Threonine 149 phosphorylation enhances δFosB transcriptional activity to control psychomotor responses to cocaine. J Neurosci 34:11461–11469. 10.1523/JNEUROSCI.1611-14.2014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
