Tumor-reprogrammed resident T cells resist radiation to control tumors
- PMID: 31477729
- PMCID: PMC6718618
- DOI: 10.1038/s41467-019-11906-2
Tumor-reprogrammed resident T cells resist radiation to control tumors
Abstract
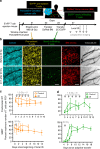
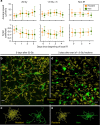
Successful combinations of radiotherapy and immunotherapy depend on the presence of live T cells within the tumor; however, radiotherapy is believed to damage T cells. Here, based on longitudinal in vivo imaging and functional analysis, we report that a large proportion of T cells survive clinically relevant doses of radiation and show increased motility, and higher production of interferon gamma, compared with T cells from unirradiated tumors. Irradiated intratumoral T cells can mediate tumor control without newly-infiltrating T cells. Transcriptomic analysis suggests T cell reprogramming in the tumor microenvironment and similarities with tissue-resident memory T cells, which are more radio-resistant than circulating/lymphoid tissue T cells. TGFβ is a key upstream regulator of T cell reprogramming and contributes to intratumoral Tcell radio-resistance. These findings have implications for the design of radio-immunotherapy trials in that local irradiation is not inherently immunosuppressive, and irradiation of multiple tumors might optimize systemic effects of radiotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Scott, C. M. Some quantitative aspects of the biological action or X and y rays. Special Report Series Vol. 223 (Medical Research Council, Great Britain, 1937).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

