Label-free Imaging of Tissue Architecture during Axolotl Peripheral Nerve Regeneration in Comparison to Functional Recovery
- PMID: 31477751
- PMCID: PMC6718386
- DOI: 10.1038/s41598-019-49067-3
Label-free Imaging of Tissue Architecture during Axolotl Peripheral Nerve Regeneration in Comparison to Functional Recovery
Erratum in
-
Author Correction: Label-Free Imaging of Tissue Architecture during Axolotl Peripheral Nerve Regeneration in Comparison to Functional Recovery.Sci Rep. 2020 Sep 15;10(1):15343. doi: 10.1038/s41598-020-72252-8. Sci Rep. 2020. PMID: 32929166 Free PMC article.
Abstract
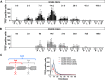
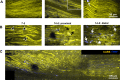
Human peripheral nerves hold the potential to regenerate after injuries; however, whether a successful axonal regrowth was achieved can be elucidated only months after injury by assessing function. The axolotl salamander is a regenerative model where nerves always regenerate quickly and fully after all types of injury. Here, de- and regeneration of the axolotl sciatic nerve were investigated in a single and double injury model by label-free multiphoton imaging in comparison to functional recovery. We used coherent anti-Stokes Raman scattering to visualize myelin fragmentation and axonal regeneration. The presence of axons at the lesion site corresponded to onset of functional recovery in both lesion models. In addition, we detected axonal regrowth later in the double injury model in agreement with a higher severity of injury. Moreover, endogenous two-photon excited fluorescence visualized macrophages and revealed a similar timecourse of inflammation in both injury models, which did not correlate with functional recovery. Finally, using the same techniques, axonal structure and status of myelin were visualized in vivo after sciatic nerve injury. Label-free imaging is a new experimental approach that provides mechanistic insights in animal models, with the potential to be used in the future for investigation of regeneration after nerve injuries in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Scholz T, et al. Peripheral Nerve Injuries: An International Survey of Current Treatments and Future Perspectives. Journal of Reconstructive Microsurgery. 2009;25:339–344. - PubMed
-
- Seddon HJ. Three types of nerve injury. Brain. 1943;66:237–288.
-
- Sunderland SYDN. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. - PubMed
-
- Palispis WA, Gupta R. Surgical repair in humans after traumatic nerve injury provides limited functional neural regeneration in adults. Exp. Neurol. 2017;290:106–114. - PubMed
-
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller’s observations to neuroinflammation. J Peripher. Nerv. Syst. 2002;7:13–27. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

