Oncolytic activity of HF10 in head and neck squamous cell carcinomas
- PMID: 31477804
- PMCID: PMC7445880
- DOI: 10.1038/s41417-019-0129-3
Oncolytic activity of HF10 in head and neck squamous cell carcinomas
Abstract
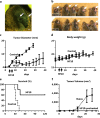
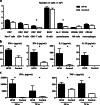
Recent developments in therapeutic strategies have improved the prognosis of head and neck squamous cell carcinoma (HNSCC). Nevertheless, 5-year survival rate remains only 40%, necessitating new therapeutic agents. Oncolytic virotherapy entails use of replication-competent viruses to selectively kill cancer cells. We aimed to explore the potential of HF10 as an oncolytic virus against human or mouse HNSCC cell lines, and primary-cultured HNSCC cells. HF10 replicated well in all the HNSCC cells, in which it induced cytopathic effects and cell killing. Next, we investigated the oncolytic effects of HF10 in ear tumor models with human or mouse tumor cells. We detected HF10-infected cells within the ear tumors based on their expression of green fluorescent protein. HF10 injection suppressed ear tumor growth and prolonged overall survival. In the syngeneic model, HF10 infection induced tumor necrosis with infiltration of CD8-positive cells. Moreover, the splenocytes of HF10-treated mice released antitumor cytokines, IL-2, IL-12, IFN-alpha, IFN-beta, IFN-gamma, and TNF-alpha, after stimulation with tumor cells in vitro. The HF10-treated mice that survived their original tumor burdens rejected tumor cells upon re-challenge. These results suggested that HF10 killed HNSCC cells and induced antitumoral immunity, thereby establishing it as a promising agent for the treatment of HNSCC patients.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev. 2011;11:9–22. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–91. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

