Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum
- PMID: 31477896
- PMCID: PMC6877471
- DOI: 10.1038/s41564-019-0539-x
Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum
Abstract
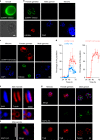
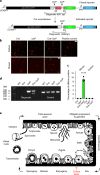
The apicomplexan parasite Cryptosporidium is a leading global cause of severe diarrhoeal disease and an important contributor to early childhood mortality. Currently, there are no fully effective treatments or vaccines available. Parasite transmission occurs through ingestion of oocysts, through either direct contact or consumption of contaminated water or food. Oocysts are meiotic spores and the product of parasite sex. Cryptosporidium has a single-host life cycle in which both asexual and sexual processes occur in the intestine of infected hosts. Here, we genetically engineered strains of Cryptosporidium to make life cycle progression and parasite sex tractable. We derive reporter strains to follow parasite development in culture and in infected mice and define the genes that orchestrate sex and oocyst formation through mRNA sequencing of sorted cells. After 2 d, parasites in cell culture show pronounced sexualization, but productive fertilization does not occur and infection falters. By contrast, in infected mice, male gametes successfully fertilize female parasites, which leads to meiotic division and sporulation. To rigorously test for fertilization, we devised a two-component genetic-crossing assay using a reporter that is activated by Cre recombinase. Our findings suggest obligate developmental progression towards sex in Cryptosporidium, which has important implications for the treatment and prevention of the infection.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Kotloff KL, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

