Regulatory T Cells: the Many Faces of Foxp3
- PMID: 31478130
- PMCID: PMC6754763
- DOI: 10.1007/s10875-019-00684-7
Regulatory T Cells: the Many Faces of Foxp3
Abstract
Regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (Foxp3) play a requisite role in the maintenance of immunological homeostasis and prevention of peripheral self-tolerance breakdown. Although Foxp3 by itself is neither necessary nor sufficient to specify many aspects of the Treg cell phenotype, its sustained expression in Treg cells is indispensable for their phenotypic stability, metabolic fitness, and regulatory function. In this review, we summarize recent advances in Treg cell biology, with a particular emphasis on the role of Foxp3 as a transcriptional modulator and metabolic gatekeeper essential to an effective immune regulatory response. We discuss these findings in the context of human inborn errors of immune dysregulation, with a focus on FOXP3 mutations, leading to Treg cell deficiency. We also highlight emerging concepts of therapeutic Treg cell reprogramming to restore tolerance in the settings of immune dysregulatory disorders.
Keywords: Autoimmunity; Foxp3; IPEX; immune dysregulation; immune tolerance; immunometabolism; interleukin 2; rapamycin; regulatory T cell reprogramming; regulatory T cells.
Conflict of interest statement
Figures


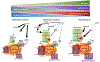

References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. - PubMed
-
- Bonomo A, Kehn PJ, Payer E, Rizzo L, Cheever AW, Shevach EM. Pathogenesis of post-thymectomy autoimmunity. Role of syngeneic MLR-reactive T cells. J Immunol. 1995;154(12):6602–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

