Present and Future of Surface-Enhanced Raman Scattering
- PMID: 31478375
- PMCID: PMC6990571
- DOI: 10.1021/acsnano.9b04224
Present and Future of Surface-Enhanced Raman Scattering
Abstract
The discovery of the enhancement of Raman scattering by molecules adsorbed on nanostructured metal surfaces is a landmark in the history of spectroscopic and analytical techniques. Significant experimental and theoretical effort has been directed toward understanding the surface-enhanced Raman scattering (SERS) effect and demonstrating its potential in various types of ultrasensitive sensing applications in a wide variety of fields. In the 45 years since its discovery, SERS has blossomed into a rich area of research and technology, but additional efforts are still needed before it can be routinely used analytically and in commercial products. In this Review, prominent authors from around the world joined together to summarize the state of the art in understanding and using SERS and to predict what can be expected in the near future in terms of research, applications, and technological development. This Review is dedicated to SERS pioneer and our coauthor, the late Prof. Richard Van Duyne, whom we lost during the preparation of this article.
Keywords: SEIRA; SERS tags; TERS; biosensing; catalysis; charge transfer; chemosensors; hot electrons; nanomedicine; surface-enhanced Raman scattering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







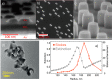

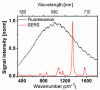

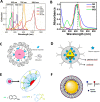
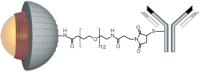















References
-
- Fleischmann M.; Hendra P. J.; McQuillan A. J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. 10.1016/0009-2614(74)85388-1. - DOI
-
- Jeanmaire D. L.; Van Duyne R. P. Surface Raman Spectroelectrochemistry Part I. Heterocyclic, Aromatic, and Aliphatic Amines Adsorbed on the Anodized Silver Electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. 10.1016/S0022-0728(77)80224-6. - DOI
-
- Albrecht M. G.; Creighton J. A. Anomalously Intense Raman Spectra of Pyridine at a Silver Electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. 10.1021/ja00457a071. - DOI
-
- Philpott M. R. Effect of Surface Plasmons on Transitions in Molecules. J. Chem. Phys. 1975, 62, 1812.10.1063/1.430708. - DOI
-
- Moskovits M. Surface Roughness and the Enhanced Intensity of Raman Scattering by Molecules Adsorbed on Metals. J. Chem. Phys. 1978, 69, 4159.10.1063/1.437095. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

