Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial
- PMID: 31478755
- PMCID: PMC7364307
- DOI: 10.1089/cap.2018.0156
Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial
Abstract
Objective: A randomized pilot trial of gastrointestinal (GI) symptoms targeting probiotic for quality of life in autism spectrum disorder (ASD). Methods: Thirteen children, 3-12 years of age with ASD, anxiety, and GI symptoms, were randomized into a probiotic crossover trial of 8 weeks each on VISBIOME and placebo separated by a 3-week washout. VISBIOME contains eight probiotic species, mostly Lactobacillus and Bifidobacterium. Primary outcome was the Pediatric Quality of Life Inventory (PedsQL) GI module. Secondary outcomes included gut microbiota analysis, the Parent-Rated Anxiety Scale for ASD (PRAS-ASD), and parent-selected target symptoms. A mixed analysis model was applied. Results: Thirteen children were randomized, with 10 completing the study (77% retention): 6 in probiotic/placebo sequence, 4 in placebo/probiotic sequence. Adherence to study treatment was 96%. There were no serious adverse events (AEs), and more nonserious AEs occurred with placebo than with probiotic, including those attributable to treatment. Only 6 of the 10 guessed the correct treatment at the end of week 8. Over the 19-week trial, each outcome improved from baseline and PedsQL correlated significantly with abundance of Lactobacillus without discernable changes to microbiota composition/diversity. Although probiotic showed more improvement than placebo, PedsQL and PRAS-ASD were not statistically significant, as expected at this sample size. PedsQL effect size was d = 0.49 by the general model and d = 0.79 by simple comparison of week 8 changes. A parent-selected target symptom showed significant improvement in GI complaints on probiotic compared with placebo (p = 0.02, d = 0.79). Probiotic effects carried over through the 3-week washout. Conclusion: The VISBIOME formulation was safe and suggested a health benefit in children with ASD and GI symptoms who retained Lactobacillus. The moderate effect size compared with placebo warrants a larger trial using a parallel-group design.
Keywords: anxiety; autism; autism spectrum disorder; gastrointestinal problems; probiotics; quality of life.
Figures
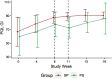

References
-
- Abidin RR: Parenting Stress Index—Short Form (3rd ed.). Odessa, FL, Psychological Assessment Resources, Inc., 1995
-
- Abidin RR: Parenting Stress Index Manual (4th ed.). Odessa, FL, Psychological Assessment Resources, 2012
-
- Aman MG, Singh NN, Stewart AW, Field CJ: The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Defic 89:485–491, 1985a - PubMed
-
- Aman MG, Singh NN, Stewart AW, Field CJ: Psychometric characteristics of the Aberrant Behavior Checklist. Am J Ment Defic 489:492–502, 1985b - PubMed
-
- Aman MG, Singh NN, Turbott SH: Reliability of the Aberrant Behavior Checklist and the effect of variations in instructions. Am J Ment Defic 92:237–240, 1987 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

