Targeting innate immunity for tuberculosis vaccination
- PMID: 31478909
- PMCID: PMC6715374
- DOI: 10.1172/JCI128877
Targeting innate immunity for tuberculosis vaccination
Abstract
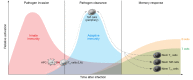
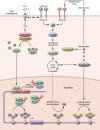
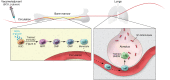
Vaccine development against tuberculosis (TB) is based on the induction of adaptive immune responses endowed with long-term memory against mycobacterial antigens. Memory B and T cells initiate a rapid and robust immune response upon encounter with Mycobacterium tuberculosis, thus achieving long-lasting protection against infection. Recent studies have shown, however, that innate immune cell populations such as myeloid cells and NK cells also undergo functional adaptation after infection or vaccination, a de facto innate immune memory that is also termed trained immunity. Experimental and epidemiological data have shown that induction of trained immunity contributes to the beneficial heterologous effects of vaccines such as bacille Calmette-Guérin (BCG), the licensed TB vaccine. Moreover, increasing evidence argues that trained immunity also contributes to the anti-TB effects of BCG vaccination. An interaction among immunological signals, metabolic rewiring, and epigenetic reprogramming underlies the molecular mechanisms mediating trained immunity in myeloid cells and their bone marrow progenitors. Future studies are warranted to explore the untapped potential of trained immunity to develop a future generation of TB vaccines that would combine innate and adaptive immune memory induction.
Conflict of interest statement
Figures