Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis
- PMID: 31479239
- PMCID: PMC7169949
- DOI: 10.1021/acsnano.9b04397
Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis
Abstract
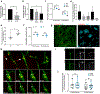
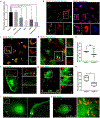
The restrictive nature of the blood-brain barrier (BBB) creates a major challenge for brain drug delivery with current nanomedicines lacking the ability to cross the BBB. Extracellular vesicles (EVs) have been shown to contribute to the progression of a variety of brain diseases including metastatic brain cancer and have been suggested as promising therapeutics and drug delivery vehicles. However, the ability of native tumor-derived EVs to breach the BBB and the mechanism(s) involved in this process remain unknown. Here, we demonstrate that tumor-derived EVs can breach the intact BBB in vivo, and by using state-of-the-art in vitro and in vivo models of the BBB, we have identified transcytosis as the mechanism underlying this process. Moreover, high spatiotemporal resolution microscopy demonstrated that the endothelial recycling endocytic pathway is involved in this transcellular transport. We further identify and characterize the mechanism by which tumor-derived EVs circumvent the low physiologic rate of transcytosis in the BBB by decreasing the brain endothelial expression of rab7 and increasing the efficiency of their transport. These findings identify previously unknown mechanisms by which tumor-derived EVs breach an intact BBB during the course of brain metastasis and can be leveraged to guide and inform the development of drug delivery approaches to deliver therapeutic cargoes across the BBB for treatment of a variety of brain diseases including, but not limited to, brain malignancies.
Keywords: blood−brain barrier; brain metastasis; breast cancer; drug delivery; exosomes; extracellular vesicles; transcytosis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tang W; Fan W; Lau J; Deng L; Shen Z; Chen X Emerging Blood-Brain-Barrier-Crossing Nanotechnology for Brain Cancer Theranostics. Chem. Soc. Rev 2019, 48, 2967–3014. - PubMed
-
- Abbott NJ; Ronnback L; Hansson E Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci 2006, 7, 41–53. - PubMed
-
- Armulik A; Genove G; Mae M; Nisancioglu MH; Wallgard E; Niaudet C; He L; Norlin J; Lindblom P; Strittmatter K; Johansson BR; Betsholtz C Pericytes Regulate the Blood-Brain Barrier. Nature 2010, 468, 557–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

