Soluble epoxide hydrolase promotes astrocyte survival in retinopathy of prematurity
- PMID: 31479425
- PMCID: PMC6877309
- DOI: 10.1172/JCI123835
Soluble epoxide hydrolase promotes astrocyte survival in retinopathy of prematurity
Abstract
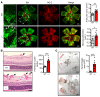
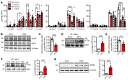
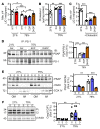
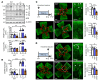
Polyunsaturated fatty acids such as docosahexaenoic acid (DHA) positively affect the outcome of retinopathy of prematurity (ROP). Given that DHA metabolism by cytochrome P450 and soluble epoxide hydrolase (sEH) enzymes affects retinal angiogenesis and vascular stability, we investigated the role of sEH in a mouse model of ROP. In WT mice, hyperoxia elicited tyrosine nitration and inhibition of sEH and decreased generation of the DHA-derived diol 19,20-dihydroxydocosapentaenoic acid (19,20-DHDP). Correspondingly, in a murine model of ROP, sEH-/- mice developed a larger central avascular zone and peripheral pathological vascular tuft formation than did their WT littermates. Astrocytes were the cells most affected by sEH deletion, and hyperoxia increased astrocyte apoptosis. In rescue experiments, 19,20-DHDP prevented astrocyte loss by targeting the mitochondrial membrane to prevent the hyperoxia-induced dissociation of presenilin-1 and presenilin-1-associated protein to attenuate poly ADP-ribose polymerase activation and mitochondrial DNA damage. Therapeutic intravitreal administration of 19,20-DHDP not only suppressed astrocyte loss, but also reduced pathological vascular tuft formation in sEH-/- mice. Our data indicate that sEH activity is required for mitochondrial integrity and retinal astrocyte survival in ROP. Moreover, 19,20-DHDP may be more effective than DHA as a nutritional supplement for preventing retinopathy in preterm infants.
Keywords: Angiogenesis; Apoptosis; Eicosanoids; Ophthalmology; Retinopathy.
Conflict of interest statement
Figures






References
-
- Lapillonne A, Moltu SJ. Long-chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann Nutr Metab. 2016;69(suppl 1):35–44. - PubMed
-
- Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 suppl):1467S–1476S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

