NME5 frameshift variant in Alaskan Malamutes with primary ciliary dyskinesia
- PMID: 31479451
- PMCID: PMC6743793
- DOI: 10.1371/journal.pgen.1008378
NME5 frameshift variant in Alaskan Malamutes with primary ciliary dyskinesia
Abstract
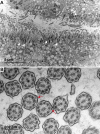
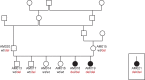
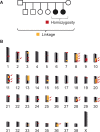
Primary ciliary dyskinesia (PCD) is a hereditary defect of motile cilia in humans and several domestic animal species. Typical clinical findings are chronic recurrent infections of the respiratory tract and fertility problems. We analyzed an Alaskan Malamute family, in which two out of six puppies were affected by PCD. The parents were unaffected suggesting autosomal recessive inheritance. Linkage and homozygosity mapping defined critical intervals comprising ~118 Mb. Whole genome sequencing of one case and comparison to 601 control genomes identified a disease associated frameshift variant, c.43delA, in the NME5 gene encoding a sparsely characterized protein associated with ciliary function. Nme5-/- knockout mice exhibit doming of the skull, hydrocephalus and sperm flagellar defects. The genotypes at NME5:c.43delA showed the expected co-segregation with the phenotype in the Alaskan Malamute family. An additional unrelated Alaskan Malamute with PCD and hydrocephalus that became available later in the study was also homozygous mutant at the NME5:c.43delA variant. The mutant allele was not present in more than 1000 control dogs from different breeds. Immunohistochemistry demonstrated absence of the NME5 protein from nasal epithelia of an affected dog. We therefore propose NME5:c.43delA as the most likely candidate causative variant for PCD in Alaskan Malamutes. These findings enable genetic testing to avoid the unintentional breeding of affected dogs in the future. Furthermore, the results of this study identify NME5 as a novel candidate gene for unsolved human PCD and/or hydrocephalus cases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Kartagener M. Zur Pathogenese der Bronchiektasien: Bronchiektasien bei Situs viscerum inversus. Beiträge zur Klinik der Tuberkulose. 1933;83(4):498–501.

