National survey of pre-treatment HIV drug resistance in Cuban patients
- PMID: 31479466
- PMCID: PMC6719847
- DOI: 10.1371/journal.pone.0221879
National survey of pre-treatment HIV drug resistance in Cuban patients
Abstract
Background: The World Health Organization (WHO) recommends a method to estimate nationally representative pretreatment HIV drug resistance (PDR) in order to evaluate the effectiveness of first -line treatments. The objective of the present study was to determine the prevalence of PDR in Cuban adults infected with HIV-1.
Materials and methods: A cross-sectional study in Cuban adults infected with HIV-1 over 18 years was conducted. The probability proportional to size method for the selection of municipalities and patients without a prior history of antiretroviral treatment during the period from January 2017 to June 2017 was used. The plasma from 141 patients from 15 municipalities for the determination of viral subtype and HIV drug resistance was collected. Some clinical and epidemiological variables were evaluated.
Results: 80. 9% of the patients corresponded to the male sex and 76.3% were men who have sex with other men (MSM). The median CD4 count was 371 cells / mm3 and the median viral load was 68000 copies / mL. The predominant genetic variants were subtype B (26.9%), CRF19_cpx (24.1%), CRF 20, 23, 24_BG (23.4%) and CRF18_cpx (12%). Overall, the prevalence of PDR was 29.8% (95%, CI 22.3-38.1). The prevalence was 12.8% (95%, CI 6.07-16.9) for any nucleoside reverse transcriptase inhibitor (NRTI), 23.4% (95%, CI 16.7-31.3) for any non-reverse transcriptase inhibitor (NNRTI) and 1.4% (95%, CI 0.17-5.03) for any protease inhibitor (PI). The most frequent mutations detected were K103N (12.9%), G190A (6.4%) and Y181C (4.8%).
Conclusions: The NNRTI prevalence above 10% in our study indicates that the first-line antiretroviral therapy in Cuba may be less effective and supports the need to look for new treatment options that contribute to therapeutic success and help the country achieve the global goals 90-90-90 set forth by UNAIDS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
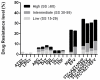
Similar articles
-
Diversity of HIV-1 genotypes and high prevalence of pretreatment drug resistance in newly diagnosed HIV-infected patients in Shanghai, China.BMC Infect Dis. 2019 Apr 8;19(1):313. doi: 10.1186/s12879-019-3927-1. BMC Infect Dis. 2019. PMID: 30961560 Free PMC article.
-
Antiretroviral drug resistance in HIV-1 therapy-naive patients in Cuba.Infect Genet Evol. 2013 Jun;16:144-50. doi: 10.1016/j.meegid.2013.02.002. Epub 2013 Feb 14. Infect Genet Evol. 2013. PMID: 23416260
-
Survey of Pretreatment HIV Drug Resistance and Genetic Transmission Network Analysis Among HIV Patients in a High Drug-Use Area of Southwest China.Curr HIV Res. 2019;17(6):441-451. doi: 10.2174/1570162X17666191128101426. Curr HIV Res. 2019. PMID: 31778107 Free PMC article.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Prevalence of pretreatment HIV drug resistance in key populations: a systematic review and meta-analysis.J Int AIDS Soc. 2020 Dec;23(12):e25656. doi: 10.1002/jia2.25656. J Int AIDS Soc. 2020. PMID: 33369131 Free PMC article.
Cited by
-
High Level of Pretreatment and Acquired Human Immunodeficiency Virus Drug Resistance in El Salvador: A Nationally Representative Survey, 2018-2019.Open Forum Infect Dis. 2022 Nov 3;9(11):ofac580. doi: 10.1093/ofid/ofac580. eCollection 2022 Nov. Open Forum Infect Dis. 2022. PMID: 36438615 Free PMC article.
-
Cuban history of CRF19 recombinant subtype of HIV-1.PLoS Pathog. 2021 Aug 9;17(8):e1009786. doi: 10.1371/journal.ppat.1009786. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34370795 Free PMC article.
-
HIV-1 Unique Recombinant Forms Identified in Slovenia and Their Characterization by Near Full-Length Genome Sequencing.Viruses. 2020 Jan 3;12(1):63. doi: 10.3390/v12010063. Viruses. 2020. PMID: 31947872 Free PMC article.
-
Pretreatment and acquired HIV drug resistance in Belize-results of nationally representative surveys, 2021-22.J Antimicrob Chemother. 2025 Jan 3;80(1):292-300. doi: 10.1093/jac/dkae408. J Antimicrob Chemother. 2025. PMID: 39556256
-
HIV Drug Resistance in Adults Initiating or Reinitiating Antiretroviral Therapy in Uruguay-Results of a Nationally Representative Survey, 2018-2019.Viruses. 2023 Feb 10;15(2):490. doi: 10.3390/v15020490. Viruses. 2023. PMID: 36851704 Free PMC article.
References
-
- Bennett D, Myatt M, Bertagnolio S, Sutherland D, Gilks C. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antiviral Therapy 2008; 13 (Supl.2): 25–36. - PubMed
-
- UNAIDS Report on the Global AIDS epidemic, 2015. Disponible en: http://www.unaids.org/globalreport/default_en.htm. UNAIDS/JC2502/1/E*. ISBN 978-92-9253-032-7.
-
- WHO 2014. Surveillance of HIV drug resistance in populations initiating antiretroviral therapy (pre-treatment HIV drug resistance). ISBN 978 92 4 1507 196.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

