Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER-3 study
- PMID: 31479549
- PMCID: PMC7028252
- DOI: 10.1111/jdv.15921
Ixekizumab sustains high level of efficacy and favourable safety profile over 4 years in patients with moderate psoriasis: results from UNCOVER-3 study
Abstract
Background: Psoriasis, a chronic disease usually requires long-term disease management.
Objective: This study evaluates the efficacy and safety of recommended ixekizumab (IXE) dose over 4 years (204 weeks) from UNCOVER-3 study.
Methods: UNCOVER-3 was a randomised, double-blind, multicenter, phase 3 study wherein patients with moderate-to-severe plaque psoriasis received placebo, IXE 80 mg every 2 weeks (Q2W), IXE 80 mg every 4 weeks (Q4W) (both IXE groups had 160 mg starting dose) or etanercept 50 mg twice weekly. At week 12, all patients switched to IXE Q4W dose for the long-term extension (264 weeks). After week 60 and at investigator's discretion, patients could receive dose adjustment to IXE Q2W. The efficacy endpoints at week 204 were percentage of patients achieving PASI 75/90/100, sPGA score of 1 or 0, and those achieving PSSI = 0, NAPSI = 0 and PPASI 100. Efficacy data were summarised through 204 weeks using as-observed, multiple imputation (MI) and modified non-responder imputation (mNRI) methods.
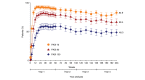
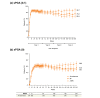
Results: The proportion of patients achieving PASI 75/90/100 at week 204 using mNRI method were 82.8%, 66.4% and 48.3%, respectively. Using as-observed and MI methods, 98.2% and 94.8% patients achieved PASI 75, 87.8% and 73.3% achieved PASI 90, and 67.1% and 52.7% achieved PASI 100 response, respectively, at week 204. The response rates for sPGA (0, 1) were 88.7%, 76.2% and 68.5% and for sPGA (0) were 68.9%, 54.6% and 49.7% using as-observed, MI and mNRI methods, respectively. Similar trends were observed with NAPSI = 0, PSSI = 0, PPASI 100 and itch NRS = 0. There were no new safety concerns through year 4.
Conclusions: This study demonstrated sustained high-efficacy response through 4 years of continuous treatment with ixekizumab in patients with moderate-to-severe plaque psoriasis. The safety profile remained consistent with prior findings, with no new or unexpected safety concerns.
© 2019 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures


References
-
- Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. - PubMed
-
- Gordon KB, Blauvelt A, Papp KA et al Phase 3 trials of ixekizumab in moderate‐to‐severe plaque psoriasis. N Engl J Med 2016; 375: 345–356. - PubMed
-
- Strober B, Leonardi C, Papp KA et al Short‐ and long‐term safety outcomes with ixekizumab from 7 clinical trials in psoriasis: etanercept comparisons and integrated data. J Am Acad Dermatol 2017; 76: 432–440.e417. - PubMed
-
- Blauvelt A, Gooderham M, Iversen L et al Efficacy and safety of ixekizumab for the treatment of moderate‐to‐severe plaque psoriasis: results through 108 weeks of a randomized, controlled phase 3 clinical trial (UNCOVER‐3). J Am Acad Dermatol 2017; 77: 855–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

