Omega-3 Docosahexaenoic Acid Is a Mediator of Fate-Decision of Adult Neural Stem Cells
- PMID: 31480215
- PMCID: PMC6747551
- DOI: 10.3390/ijms20174240
Omega-3 Docosahexaenoic Acid Is a Mediator of Fate-Decision of Adult Neural Stem Cells
Abstract
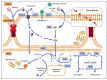
The mammalian brain is enriched with lipids that serve as energy catalyzers or secondary messengers of essential signaling pathways. Docosahexaenoic acid (DHA) is an omega-3 fatty acid synthesized de novo at low levels in humans, an endogenous supply from its precursors, and is mainly incorporated from nutrition, an exogeneous supply. Decreased levels of DHA have been reported in the brains of patients with neurodegenerative diseases. Preventing this decrease or supplementing the brain with DHA has been considered as a therapy for the DHA brain deficiency that could be linked with neuronal death or neurodegeneration. The mammalian brain has, however, a mechanism of compensation for loss of neurons in the brain: neurogenesis, the birth of neurons from neural stem cells. In adulthood, neurogenesis is still present, although at a slower rate and with low efficiency, where most of the newly born neurons die. Neural stem/progenitor cells (NSPCs) have been shown to require lipids for proper metabolism for proliferation maintenance and neurogenesis induction. Recent studies have focused on the effects of these essential lipids on the neurobiology of NSPCs. This review aimed to introduce the possible use of DHA to impact NSPC fate-decision as a therapy for neurodegenerative diseases.
Keywords: adult neurogenesis; docosahexaenoic acid; neural stem cell; neuroprotection; omega-3 fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeting the Brain with a Neuroprotective Omega-3 Fatty Acid to Enhance Neurogenesis in Hypoxic Condition in Culture.Mol Neurobiol. 2019 Feb;56(2):986-999. doi: 10.1007/s12035-018-1139-0. Epub 2018 Jun 1. Mol Neurobiol. 2019. PMID: 29858775
-
Distinctive effects of arachidonic acid and docosahexaenoic acid on neural stem /progenitor cells.Genes Cells. 2011 Jul;16(7):778-90. doi: 10.1111/j.1365-2443.2011.01527.x. Epub 2011 Jun 13. Genes Cells. 2011. PMID: 21668588
-
Docosahexaenoic acid (DHA) enhances the therapeutic potential of neonatal neural stem cell transplantation post-Traumatic brain injury.Behav Brain Res. 2018 Mar 15;340:1-13. doi: 10.1016/j.bbr.2017.11.007. Epub 2017 Nov 7. Behav Brain Res. 2018. PMID: 29126932
-
Effects of addictive drugs on adult neural stem/progenitor cells.Cell Mol Life Sci. 2016 Jan;73(2):327-48. doi: 10.1007/s00018-015-2067-z. Epub 2015 Oct 14. Cell Mol Life Sci. 2016. PMID: 26468052 Free PMC article. Review.
-
Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle.Nutrients. 2021 Mar 18;13(3):986. doi: 10.3390/nu13030986. Nutrients. 2021. PMID: 33803760 Free PMC article. Review.
Cited by
-
Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system.Front Psychiatry. 2022 Aug 31;13:933704. doi: 10.3389/fpsyt.2022.933704. eCollection 2022. Front Psychiatry. 2022. PMID: 36117650 Free PMC article. Review.
-
Investigation of Lysophospholipids-DHA transport across an in vitro human model of blood brain barrier.Heliyon. 2024 Oct 2;10(19):e38871. doi: 10.1016/j.heliyon.2024.e38871. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39421371 Free PMC article.
-
Emerging Role of Phospholipids and Lysophospholipids for Improving Brain Docosahexaenoic Acid as Potential Preventive and Therapeutic Strategies for Neurological Diseases.Int J Mol Sci. 2022 Apr 2;23(7):3969. doi: 10.3390/ijms23073969. Int J Mol Sci. 2022. PMID: 35409331 Free PMC article. Review.
-
Dolphin CONTINUE: a multi-center randomized controlled trial to assess the effect of a nutritional intervention on brain development and long-term outcome in infants born before 30 weeks of gestation.BMC Pediatr. 2024 Jun 7;24(1):384. doi: 10.1186/s12887-024-04849-1. BMC Pediatr. 2024. PMID: 38849784 Free PMC article. Clinical Trial.
-
Influences of Diet Quality, Nursery-Habitat Complexity and Sex on Brain Development and Cognitive Performance of Brown Trout (Salmo trutta L.).Ecol Evol. 2025 Aug 25;15(8):e71924. doi: 10.1002/ece3.71924. eCollection 2025 Aug. Ecol Evol. 2025. PMID: 40873731 Free PMC article.
References
-
- Moreno-Jiménez E.P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N., Ávila J., Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

