Physicochemical Properties of A New PEGylated Polybenzofulvene Brush for Drug Encapsulation
- PMID: 31480633
- PMCID: PMC6781277
- DOI: 10.3390/pharmaceutics11090444
Physicochemical Properties of A New PEGylated Polybenzofulvene Brush for Drug Encapsulation
Abstract
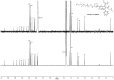
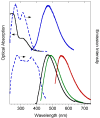
A new polymer brush was synthesized by spontaneous polymerization of benzofulvene macromonomer 6-MOEG-9-T-BF3k bearing a nona(ethylene glycol) side chain linked to the 3-phenylindene scaffold by means of a triazole heterocycle. The polymer structure was studied by SEC-MALS, NMR spectroscopy, and MALDI-TOF MS techniques, and the results supported the role of oligomeric initiatory species in the spontaneous polymerization of polybenzofulvene derivatives. The aggregation features of high molecular weight poly-6-MOEG-9-T-BF3k-FE were investigated by pyrene fluorescence analysis, dynamic light scattering studies, and transmission electron microscopy, which suggested a tendency towards the formation of spherical objects showing dimensions in the range of 20-200 nm. Moreover, poly-6-MOEG-9-T-BF3k-FE showed an interesting cytocompatibility in the whole concentration range tested that, besides its aggregation features, makes this polybenzofulvene brush a good polymer candidate for nanoencapsulation and delivery of drug molecules. Finally, the photo-physical features of poly-6-MOEG-9-T-BF3k-FE could allow the biodistribution of the resulting drug delivery systems to be monitored by fluorescence microscopy techniques.
Keywords: PEGylation; affinity polymerization; drug delivery systems; grafting through; nanocarrier; polybenzofulvene; spontaneous polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Polymeropoulos G., Zapsas G., Ntetsikas K., Bilalis P., Gnanou Y., Hadjichristidis N. 50th Anniversary Perspective: Polymers with Complex Architectures. Macromolecules. 2017;50:1253–1290. doi: 10.1021/acs.macromol.6b02569. - DOI
-
- Zhang M., Müller A.H.E. Cylindrical polymer brushes. J. Polym. Sci. Part A Polym. Chem. 2005;43:3461–3481. doi: 10.1002/pola.20900. - DOI
-
- Sheiko S.S., Sumerlin B.S., Matyjaszewski K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008;33:759–785. doi: 10.1016/j.progpolymsci.2008.05.001. - DOI
-
- Neiser M.W., Okuda J., Schmidt M. Polymerization of macromonomers to cylindrical brushes initiated by organolanthanides. Macromolecules. 2003;36:5437–5439. doi: 10.1021/ma034196+. - DOI
-
- Gan W., Shi Y., Jing B., Cao X., Zhu Y., Gao H. Produce Molecular Brushes with Ultrahigh Grafting Density Using Accelerated CuAAC Grafting-Onto Strategy. Macromolecules. 2017;50:215–222. doi: 10.1021/acs.macromol.6b02388. - DOI
LinkOut - more resources
Full Text Sources

