Altered excitability of small cutaneous nerve fibers during cooling assessed with the perception threshold tracking technique
- PMID: 31481024
- PMCID: PMC6724327
- DOI: 10.1186/s12868-019-0527-3
Altered excitability of small cutaneous nerve fibers during cooling assessed with the perception threshold tracking technique
Abstract
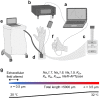
Background: There is a need for new approaches to increase the knowledge of the membrane excitability of small nerve fibers both in healthy subjects, as well as during pathological conditions. Our research group has previously developed the perception threshold tracking technique to indirectly assess the membrane properties of peripheral small nerve fibers. In the current study, a new approach for studying membrane excitability by cooling small fibers, simultaneously with applying a slowly increasing electrical stimulation current, is evaluated. The first objective was to examine whether altered excitability during cooling could be detected by the perception threshold tracking technique. The second objective was to computationally model the underlying ionic current that could be responsible for cold induced alteration of small fiber excitability. The third objective was to evaluate whether computational modelling of cooling and electrical simulation can be used to generate hypotheses of ionic current changes in small fiber neuropathy.
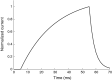
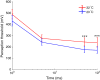
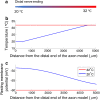
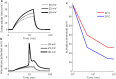
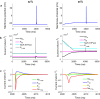
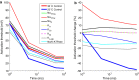
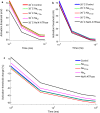
Results: The excitability of the small fibers was assessed by the perception threshold tracking technique for the two temperature conditions, 20 °C and 32 °C. A detailed multi-compartment model was developed, including the ionic currents: NaTTXs, NaTTXr, NaP, KDr, KM, KLeak, KA, and Na/K-ATPase. The perception thresholds for the two long duration pulses (50 and 100 ms) were reduced when the skin temperature was lowered from 32 to 20 °C (p < 0.001). However, no significant effects were observed for the shorter durations (1 ms, p = 0.116; 5 ms p = 0.079, rmANOVA, Sidak). The computational model predicted that the reduction in the perception thresholds related to long duration pulses may originate from a reduction of the KLeak channel and the Na/K-ATPase. For short durations, the effect cancels out due to a reduction of the transient TTX resistant sodium current (Nav1.8). Additionally, the result from the computational model indicated that cooling simultaneously with electrical stimulation, may increase the knowledge regarding pathological alterations of ionic currents.
Conclusion: Cooling may alter the ionic current during electrical stimulation and thereby provide additional information regarding membrane excitability of small fibers in healthy subjects and potentially also during pathological conditions.
Keywords: Electrical stimulation; Multicompartment model; Na/K-ATPase; Perception threshold tracking; Temperature; Voltage-gated ion channels.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Bromm B, Meier W. The intracutaneous stimulus—a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol. 1984;6:405–410. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

