An algorithm for the classification of study designs to assess diagnostic, prognostic and predictive test accuracy in systematic reviews
- PMID: 31481098
- PMCID: PMC6720081
- DOI: 10.1186/s13643-019-1131-4
An algorithm for the classification of study designs to assess diagnostic, prognostic and predictive test accuracy in systematic reviews
Abstract
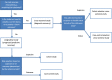
Results of medical tests are the main source to inform clinical decision making. The main information to assess the usefulness of medical tests for correct discrimination of patients are accuracy measures. For the estimation of test accuracy measures, many different study designs can be used. The study design is related to the clinical question to be answered (diagnosis, prognosis, prediction), determines the accuracy measures that can be calculated and it might have an influence on risk of bias. Therefore, a clear and consistent distinction of the different study designs in systematic reviews on test accuracy studies is very important. In this paper, we propose an algorithm for the classification of study designs of test accuracy, that compare the results of an index test (the test to be evaluated) with the results of a reference test (the test whose results are considered as correct/the gold standard) studies in systematic reviews.
Keywords: Diagnosis prognosis; Diagnostic accuracy; Prediction; Sensitivity; Specificity; Study design classification.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, Scholten R, Langendam M, Leeflang MM, Akl EA, et al. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J Clin Epidemiol. 2016;76(Supplement C):89–98. doi: 10.1016/j.jclinepi.2016.01.032. - DOI - PubMed
-
- Bae JH, Park SH, Ye BD, Kim SO, Cho YK, Youn EJ, Lee HS, Hwang SW, Yang DH, Kim KJ, et al. Development and validation of a novel prediction model for differential diagnosis between Crohn's disease and intestinal tuberculosis. Inflamm Bowel Dis. 2017;23(9):1614–1623. doi: 10.1097/MIB.0000000000001162. - DOI - PubMed
-
- Giannini V, Mazzetti S, Marmo A, Montemurro F, Regge D, Martincich L. A computer-aided diagnosis (CAD) scheme for pretreatment prediction of pathological response to neoadjuvant therapy using dynamic contrast-enhanced MRI texture features. Br J Radiol. 2017;90(1077):20170269. doi: 10.1259/bjr.20170269. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources