Yersiniabactin-Producing Adherent/Invasive Escherichia coli Promotes Inflammation-Associated Fibrosis in Gnotobiotic Il10-/- Mice
- PMID: 31481410
- PMCID: PMC6803345
- DOI: 10.1128/IAI.00587-19
Yersiniabactin-Producing Adherent/Invasive Escherichia coli Promotes Inflammation-Associated Fibrosis in Gnotobiotic Il10-/- Mice
Abstract
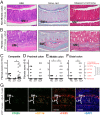
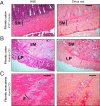
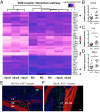
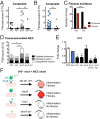
Fibrosis is a significant complication of intestinal disorders associated with microbial dysbiosis and pathobiont expansion, notably Crohn's disease (CD). Mechanisms that favor fibrosis are not well understood, and therapeutic strategies are limited. Here we demonstrate that colitis-susceptible Il10-deficient mice develop inflammation-associated fibrosis when monoassociated with adherent/invasive Escherichia coli (AIEC) that harbors the yersiniabactin (Ybt) pathogenicity island. Inactivation of Ybt siderophore production in AIEC nearly abrogated fibrosis development in inflamed mice. In contrast, inactivation of Ybt import through its cognate receptor FyuA enhanced fibrosis severity. This corresponded with increased colonic expression of profibrogenic genes prior to the development of histological disease, therefore suggesting causality. fyuA-deficient AIEC also exhibited greater localization within subepithelial tissues and fibrotic lesions that was dependent on Ybt biosynthesis and corresponded with increased fibroblast activation in vitro Together, these findings suggest that Ybt establishes a profibrotic environment in the host in the absence of binding to its cognate receptor and indicate a direct link between intestinal AIEC and the induction of inflammation-associated fibrosis.
Keywords: AIEC; Crohn’s disease; colitis; fibrosis; intestinal inflammation; microbiome; microbiota; yersiniabactin.
Copyright © 2019 Ellermann et al.
Figures



Similar articles
-
Intestinal E. coli-produced yersiniabactin promotes profibrotic macrophages in Crohn's disease.Cell Host Microbe. 2025 Jan 8;33(1):71-88.e9. doi: 10.1016/j.chom.2024.11.012. Epub 2024 Dec 18. Cell Host Microbe. 2025. PMID: 39701098
-
Siderophore Immunization Restricted Colonization of Adherent-Invasive Escherichia coli and Ameliorated Experimental Colitis.mBio. 2022 Oct 26;13(5):e0218422. doi: 10.1128/mbio.02184-22. Epub 2022 Sep 12. mBio. 2022. PMID: 36094114 Free PMC article.
-
Yersiniabactin Siderophore of Crohn's Disease-Associated Adherent-Invasive Escherichia coli Is Involved in Autophagy Activation in Host Cells.Int J Mol Sci. 2021 Mar 29;22(7):3512. doi: 10.3390/ijms22073512. Int J Mol Sci. 2021. PMID: 33805299 Free PMC article.
-
Understanding host-adherent-invasive Escherichia coli interaction in Crohn's disease: opening up new therapeutic strategies.Biomed Res Int. 2014;2014:567929. doi: 10.1155/2014/567929. Epub 2014 Dec 15. Biomed Res Int. 2014. PMID: 25580435 Free PMC article. Review.
-
The role of major virulence factors and pathogenicity of adherent-invasive Escherichia coli in patients with Crohn's disease.Prz Gastroenterol. 2020;15(4):279-288. doi: 10.5114/pg.2020.93235. Epub 2020 Dec 10. Prz Gastroenterol. 2020. PMID: 33777266 Free PMC article. Review.
Cited by
-
A nadA Mutation Confers Nicotinic Acid Auxotrophy in Pro-carcinogenic Intestinal Escherichia coli NC101.Front Microbiol. 2021 Jun 2;12:670005. doi: 10.3389/fmicb.2021.670005. eCollection 2021. Front Microbiol. 2021. PMID: 34149655 Free PMC article.
-
Heterogeneity among Clinical Intestinal Escherichia coli Isolates upon Acquired Streptomycin Resistance.Microbiol Spectr. 2023 Jun 15;11(3):e0350022. doi: 10.1128/spectrum.03500-22. Epub 2023 May 15. Microbiol Spectr. 2023. PMID: 37184392 Free PMC article.
-
Yersiniabactin produced by Escherichia coli promotes intestinal inflammation through lipid peroxidation and ferroptosis.Front Microbiol. 2025 Feb 17;16:1542801. doi: 10.3389/fmicb.2025.1542801. eCollection 2025. Front Microbiol. 2025. PMID: 40034497 Free PMC article.
-
Aging-Associated Augmentation of Gut Microbiome Virulence Capability Drives Sepsis Severity.mBio. 2023 Jun 27;14(3):e0005223. doi: 10.1128/mbio.00052-23. Epub 2023 Apr 27. mBio. 2023. PMID: 37102874 Free PMC article.
-
Multiomics reveals microbial metabolites as key actors in intestinal fibrosis in Crohn's disease.EMBO Mol Med. 2024 Oct;16(10):2427-2449. doi: 10.1038/s44321-024-00129-8. Epub 2024 Sep 13. EMBO Mol Med. 2024. PMID: 39271960 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

