Probabilistic Representation in Human Visual Cortex Reflects Uncertainty in Serial Decisions
- PMID: 31481435
- PMCID: PMC6786811
- DOI: 10.1523/JNEUROSCI.3212-18.2019
Probabilistic Representation in Human Visual Cortex Reflects Uncertainty in Serial Decisions
Abstract
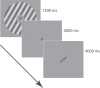
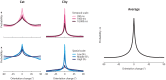
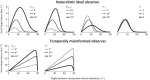
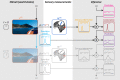
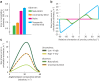
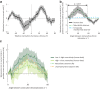
How does the brain represent the reliability of its sensory evidence? Here, we test whether sensory uncertainty is encoded in cortical population activity as the width of a probability distribution, a hypothesis that lies at the heart of Bayesian models of neural coding. We probe the neural representation of uncertainty by capitalizing on a well-known behavioral bias called serial dependence. Human observers of either sex reported the orientation of stimuli presented in sequence, while activity in visual cortex was measured with fMRI. We decoded probability distributions from population-level activity and found that serial dependence effects in behavior are consistent with a statistically advantageous sensory integration strategy, in which uncertain sensory information is given less weight. More fundamentally, our results suggest that probability distributions decoded from human visual cortex reflect the sensory uncertainty that observers rely on in their decisions, providing critical evidence for Bayesian theories of perception.SIGNIFICANCE STATEMENT Virtually any decision that people make is based on uncertain and incomplete information. Although uncertainty plays a major role in decision-making, we have but a nascent understanding of its neural basis. Here, we probe the neural code of uncertainty by capitalizing on a well-known perceptual illusion. We developed a computational model to explain the illusion, and tested it in behavioral and neuroimaging experiments. This revealed that the illusion is not a mistake of perception, but rather reflects a rational decision under uncertainty. No less important, we discovered that the uncertainty that people use in this decision is represented in brain activity as the width of a probability distribution, providing critical evidence for current Bayesian theories of decision-making.
Keywords: computational modeling; fMRI; perceptual decision-making; serial dependence; uncertainty.
Copyright © 2019 the authors.
Figures
Comment in
-
Commentary: Probabilistic Representation in Human Visual Cortex Reflects Uncertainty in Serial Decisions.Front Hum Neurosci. 2020 Oct 21;14:580581. doi: 10.3389/fnhum.2020.580581. eCollection 2020. Front Hum Neurosci. 2020. PMID: 33192413 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
