Anti-HIV and Anti-Hepatitis C Virus Drugs Inhibit P-Glycoprotein Efflux Activity in Caco-2 Cells and Precision-Cut Rat and Human Intestinal Slices
- PMID: 31481446
- PMCID: PMC6811448
- DOI: 10.1128/AAC.00910-19
Anti-HIV and Anti-Hepatitis C Virus Drugs Inhibit P-Glycoprotein Efflux Activity in Caco-2 Cells and Precision-Cut Rat and Human Intestinal Slices
Abstract
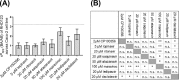
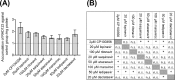
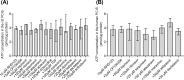
P-glycoprotein (ABCB1), an ATP-binding-cassette efflux transporter, limits intestinal absorption of its substrates and is a common site of drug-drug interactions (DDIs). ABCB1 has been suggested to interact with many antivirals used to treat HIV and/or chronic hepatitis C virus (HCV) infections. Using bidirectional transport experiments in Caco-2 cells and a recently established ex vivo model of accumulation in precision-cut intestinal slices (PCIS) prepared from rat ileum or human jejunum, we evaluated the potential of anti-HIV and anti-HCV antivirals to inhibit intestinal ABCB1. Lopinavir, ritonavir, saquinavir, atazanavir, maraviroc, ledipasvir, and daclatasvir inhibited the efflux of a model ABCB1 substrate, rhodamine 123 (RHD123), in Caco-2 cells and rat-derived PCIS. Lopinavir, ritonavir, saquinavir, and atazanavir also significantly inhibited RHD123 efflux in human-derived PCIS, while possible interindividual variability was observed in the inhibition of intestinal ABCB1 by maraviroc, ledipasvir, and daclatasvir. Abacavir, zidovudine, tenofovir disoproxil fumarate, etravirine, and rilpivirine did not inhibit intestinal ABCB1. In conclusion, using recently established ex vivo methods for measuring drug accumulation in rat- and human-derived PCIS, we have demonstrated that some antivirals have a high potential for DDIs on intestinal ABCB1. Our data help clarify the molecular mechanisms responsible for reported increases in the bioavailability of ABCB1 substrates, including antivirals and drugs prescribed to treat comorbidity. These results could help guide the selection of combination pharmacotherapies and/or suitable dosing schemes for patients infected with HIV and/or HCV.
Keywords: Caco-2 cells; P-glycoprotein; antiviral drugs; drug-drug interactions; intestinal absorption; precision-cut intestinal slices; rhodamine 123.
Copyright © 2019 American Society for Microbiology.
Figures
References
-
- World Health Organization. 2019. HIV/AIDS. http://www.who.int/hiv/data/en/. Accessed 7 February 2019.
-
- World Health Organization. 2019. Hepatitis C. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed 7 February 2019.
-
- Centers for Disease Control and Prevention. 2019. Hepatitis C kills more Americans than any other infectious disease. https://www.cdc.gov/media/releases/2016/p0504-hepc-mortality.html. Accessed 7 February 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

