Molecular interaction site on procoagulant myosin for factor Xa-dependent prothrombin activation
- PMID: 31481465
- PMCID: PMC6791324
- DOI: 10.1074/jbc.AC119.010236
Molecular interaction site on procoagulant myosin for factor Xa-dependent prothrombin activation
Abstract
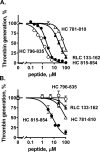
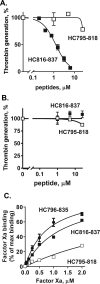
Skeletal muscle myosin has potent procoagulant activity that is based on its ability to enhance thrombin generation due to binding coagulation factors Xa and Va and accelerating prothrombin activation. A well-studied myosin inhibitor that binds to myosin's neck region inhibits myosin-dependent prothrombin activation. Hence, to identify a potential binding site(s) on skeletal muscle myosin for factor Xa, 19 peptides (25-40 residues) representing the neck region, which consists of a regulatory light chain, an essential light chain, and a heavy chain (HC), were screened for inhibition of myosin-supported prothrombin activation. Peptide HC796-835 comprising residues 796-835 of the heavy chain strongly inhibited myosin-enhanced prothrombin activation by factors Xa and Va (50% inhibition at 1.2 μm), but it did not inhibit phospholipid vesicle-enhanced prothrombin activation. Peptide inhibition studies also implicated several myosin light chain sequences located near HC796-835 as potential procoagulant sites. A peptide comprising HC796-835's C-terminal half, but not a peptide comprising its N-terminal half, inhibited myosin-enhanced prothrombin activation (50% inhibition at 1.2 μm). This inhibitory peptide (HC816-837) did not inhibit phospholipid-enhanced prothrombin activation, indicating its specificity for inhibition of myosin-dependent procoagulant mechanisms. Binding studies showed that purified factor Xa was bound to immobilized peptides HC796-835 and HC816-837 with apparent Kd values of 0.78 and 1.3 μm, respectively. In summary, these studies imply that HC residues 816-835 in the neck region of the skeletal muscle myosin directly bind factor Xa and, with contributions from light chain residues in this neck region, contribute to provision of myosin's procoagulant surface.
Keywords: coagulation factor; factor Xa; myosin; peptides; skeletal muscle; thrombin.
© 2019 Deguchi et al.
Conflict of interest statement
The Scripps Research Institute has intellectual property rights related to this study
Figures


References
-
- Deguchi H., Sinha R. K., Marchese P., Ruggeri Z. M., Zilberman-Rudenko J., McCarty O. J. T., Cohen M. J., and Griffin J. H. (2016) Prothrombotic skeletal muscle myosin directly enhances prothrombin activation by binding factors Xa and Va. Blood 128, 1870–1878 10.1182/blood-2016-03-707679 - DOI - PMC - PubMed
-
- Hayat M. D., Wyseure T., Mosnier L. O., and Griffin J. H. (2018) Skeletal muscle myosin has pro-hemostatic activity in vivo. Am. J. Hematol. 93, E25
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

