'Optimising PharmacoTherapy In the multimorbid elderly in primary CAre' (OPTICA) to improve medication appropriateness: study protocol of a cluster randomised controlled trial
- PMID: 31481568
- PMCID: PMC6731954
- DOI: 10.1136/bmjopen-2019-031080
'Optimising PharmacoTherapy In the multimorbid elderly in primary CAre' (OPTICA) to improve medication appropriateness: study protocol of a cluster randomised controlled trial
Abstract
Introduction: Multimorbidity and polypharmacy are major risk factors for potentially inappropriate prescribing (eg, overprescribing and underprescribing), and systematic medication reviews are complex and time consuming. In this trial, the investigators aim to determine if a systematic software-based medication review improves medication appropriateness more than standard care in older, multimorbid patients with polypharmacy.
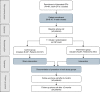
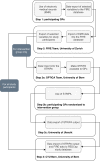
Methods and analysis: Optimising PharmacoTherapy In the multimorbid elderly in primary CAre is a cluster randomised controlled trial that will include outpatients from the Swiss primary care setting, aged ≥65 years with ≥three chronic medical conditions and concurrent use of ≥five chronic medications. Patients treated by the same general practitioner (GP) constitute a cluster, and clusters are randomised 1:1 to either a standard care sham intervention, in which the GP discusses with the patient if the medication list is complete, or a systematic medication review intervention based on the use of the 'Systematic Tool to Reduce Inappropriate Prescribing'-Assistant (STRIPA). STRIPA is a web-based clinical decision support system that helps customise medication reviews. It is based on the validated 'Screening Tool of Older Person's Prescriptions' (STOPP) and 'Screening Tool to Alert doctors to Right Treatment' (START) criteria to detect potentially inappropriate prescribing. The trial's follow-up period is 12 months. Outcomes will be assessed at baseline, 6 and 12 months. The primary endpoint is medication appropriateness, as measured jointly by the change in the Medication Appropriateness Index (MAI) and Assessment of Underutilisation (AOU). Secondary endpoints include the degree of polypharmacy, overprescribing and underprescribing, the number of falls and fractures, quality of life, the amount of formal and informal care received by patients, survival, patients' quality adjusted life years, patients' medical costs, cost-effectiveness of the intervention, percentage of recommendations accepted by GPs, percentage of recommendation rejected by GPs and patients' willingness to have medications deprescribed.
Ethics and dissemination: The ethics committee of the canton of Bern in Switzerland approved the trial protocol. The results of this trial will be published in a peer-reviewed journal.
Main funding: Swiss National Science Foundation, National Research Programme (NRP 74) 'Smarter Healthcare'.
Trial registration numbers: Clinicaltrials.gov (NCT03724539), KOFAM (Swiss national portal) (SNCTP000003060), Universal Trial Number (U1111-1226-8013).
Keywords: multimorbidity; polypharmacy; primary care.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
