Genes and Mechanisms Involved in the Generation and Amplification of Basal Radial Glial Cells
- PMID: 31481878
- PMCID: PMC6710321
- DOI: 10.3389/fncel.2019.00381
Genes and Mechanisms Involved in the Generation and Amplification of Basal Radial Glial Cells
Erratum in
-
Corrigendum: Genes and Mechanisms Involved in the Generation and Amplification of Basal Radial Glial Cells.Front Cell Neurosci. 2019 Oct 21;13:462. doi: 10.3389/fncel.2019.00462. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31695596 Free PMC article.
Abstract
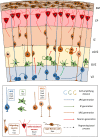
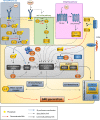
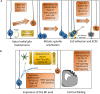
The development of the cerebral cortex relies on different types of progenitor cell. Among them, the recently described basal radial glial cell (bRG) is suggested to be of critical importance for the development of the brain in gyrencephalic species. These cells are highly numerous in primate and ferret brains, compared to lissencephalic species such as the mouse in which they are few in number. Their somata are located in basal subventricular zones in gyrencephalic brains and they generally possess a basal process extending to the pial surface. They sometimes also have an apical process directed toward the ventricular surface, similar to apical radial glial cells (aRGs) from which they are derived, and whose somata are found more apically in the ventricular zone. bRGs share similarities with aRGs in terms of gene expression (SOX2, PAX6, and NESTIN), whilst also expressing a range of more specific genes (such as HOPX). In primate brains, bRGs can divide multiple times, self-renewing and/or generating intermediate progenitors and neurons. They display a highly specific cytokinesis behavior termed mitotic somal translocation. We focus here on recently identified molecular mechanisms associated with the generation and amplification of bRGs, including bRG-like cells in the rodent. These include signaling pathways such as the FGF-MAPK cascade, SHH, PTEN/AKT, PDGF pathways, and proteins such as INSM, GPSM2, ASPM, TRNP1, ARHGAP11B, PAX6, and HIF1α. A number of these proteins were identified through transcriptome comparisons in human aRGs vs. bRGs, and validated by modifying their activities or expression levels in the mouse. This latter experiment often revealed enhanced bRG-like cell production, even in some cases generating folds (gyri) on the surface of the mouse cortex. We compare the features of the identified cells and methods used to characterize them in each model. These important data converge to indicate pathways essential for the production and expansion of bRGs, which may help us understand cortical development in health and disease.
Keywords: adhesion; basal radial glia; cell division; cortical development; neural progenitor cells; signaling pathways; spindle orientation.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

