Dissecting the Genetics of Autism Spectrum Disorders: A Drosophila Perspective
- PMID: 31481894
- PMCID: PMC6709880
- DOI: 10.3389/fphys.2019.00987
Dissecting the Genetics of Autism Spectrum Disorders: A Drosophila Perspective
Abstract
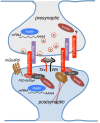
Autism Spectrum Disorder (ASD) is a complex group of multi-factorial developmental disorders that leads to communication and behavioral defects. Genetic alterations have been identified in around 20% of ASD patients and the use of genetic models, such as Drosophila melanogaster, has been of paramount importance in deciphering the significance of these alterations. In fact, many of the ASD associated genes, such as FMR1, Neurexin, Neuroligins and SHANK encode for proteins that have conserved functions in neurons and during synapse development, both in humans and in the fruit fly. Drosophila is a prominent model in neuroscience due to the conserved genetic networks that control neurodevelopmental processes and to the ease of manipulating its genetics. In the present review we will describe recent advances in the field of ASD with a particular focus on the characterization of genes where the use of Drosophila has been fundamental to better understand their function.
Keywords: Drosophila; FMR1; autism (ASD); dopamine; mGlu receptor 5; neurexin; neuroligins; shank.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

