Microglia in Retinal Degeneration
- PMID: 31481963
- PMCID: PMC6710350
- DOI: 10.3389/fimmu.2019.01975
Microglia in Retinal Degeneration
Abstract
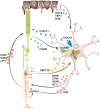
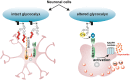
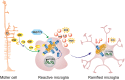
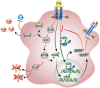
The retina is a complex tissue with multiple cell layers that are highly ordered. Its sophisticated structure makes it especially sensitive to external or internal perturbations that exceed the homeostatic range. This necessitates the continuous surveillance of the retina for the detection of noxious stimuli. This task is mainly performed by microglia cells, the resident tissue macrophages which confer neuroprotection against transient pathophysiological insults. However, under sustained pathological stimuli, microglial inflammatory responses become dysregulated, often worsening disease pathology. In this review, we provide an overview of recent studies that depict microglial responses in diverse retinal pathologies that have degeneration and chronic immune reactions as key pathophysiological components. We also discuss innovative immunomodulatory therapy strategies that dampen the detrimental immunological responses to improve disease outcome.
Keywords: chronic inflammation; immunomodulation; microglia; neuroprotection; retina.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

