Galloyl - Hexahydroxydiphenoyl (HHDP)-Glucose Isolated From Punica granatum L. Leaves Protects Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury in BALB/c Mice
- PMID: 31481965
- PMCID: PMC6710369
- DOI: 10.3389/fimmu.2019.01978
Galloyl - Hexahydroxydiphenoyl (HHDP)-Glucose Isolated From Punica granatum L. Leaves Protects Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury in BALB/c Mice
Erratum in
-
Corrigendum: Galloyl-Hexahydroxydiphenoyl (HHDP)-Glucose Isolated From Punica granatum L. Leaves Protects Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury in BALB/c Mice.Front Immunol. 2019 Nov 27;10:2727. doi: 10.3389/fimmu.2019.02727. eCollection 2019. Front Immunol. 2019. PMID: 31819743 Free PMC article.
Abstract
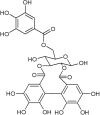
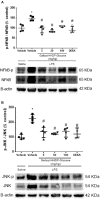
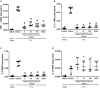
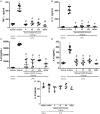
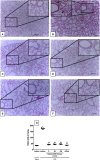
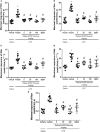
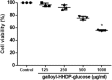
The hydroalcoholic extract and ethyl acetate fraction of Punica granatum leaves have been known to exhibit anti-inflammatory activities. In this study, we investigated the therapeutic effects of galloyl-hexahydroxydiphenoyl (HHDP)-glucose isolated from pomegranate leaves on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. Male BALB/c mice were treated with different doses of galloyl-HHDP-glucose (5, 50, and 100 mg/Kg) or dexamethasone at 5 mg/Kg (per os) 6 h after intra-tracheal instillation of LPS. Vehicle-treated mice were used as controls. Twenty-four hours after LPS challenge, bronchoalveolar lavage fluid (BALF), and lung samples were collected for analyses. They were evaluated by monitoring the expression of NF-κB, JNK, and cytokine genes and proteins, as well as cell migration and lung function. All doses of galloyl-HHDP-glucose inhibited LPS-induced JNK and NF-κB activation. Likewise, the galloyl-HHDP-glucose-treated animals presented reduced expression of the TNF-α, IL-6, and IL-1β genes in the lungs and reduced TNF-α, IL-6, IL-1β, and IL-8 protein levels when compared with the vehicle-treated LPS-challenged mice. In addition, the ALI mice treated with galloyl-HHDP-glucose also presented reduced lung inflammatory cell accumulation, especially that of neutrophils, in their BALF and lungs. In addition, galloyl-HHDP-glucose treatment markedly ameliorated the LPS-induced pulmonary mechanism complications and attenuated weight loss. Overall, we showed for the first time that galloyl-HHDP-glucose protects against ALI, and may be useful for treating ALI and other inflammatory disorders.
Keywords: acute lung injury; anti-inflammatory effects; cytokines; galloyl-HHDP-glucose; leukocytes; pomegranate.
Figures

Similar articles
-
Corrigendum: Galloyl-Hexahydroxydiphenoyl (HHDP)-Glucose Isolated From Punica granatum L. Leaves Protects Against Lipopolysaccharide (LPS)-Induced Acute Lung Injury in BALB/c Mice.Front Immunol. 2019 Nov 27;10:2727. doi: 10.3389/fimmu.2019.02727. eCollection 2019. Front Immunol. 2019. PMID: 31819743 Free PMC article.
-
Punica granatum L. Leaf Extract Attenuates Lung Inflammation in Mice with Acute Lung Injury.J Immunol Res. 2018 Feb 20;2018:6879183. doi: 10.1155/2018/6879183. eCollection 2018. J Immunol Res. 2018. PMID: 29675437 Free PMC article.
-
Total flavonoids of Mosla scabra leaves attenuates lipopolysaccharide-induced acute lung injury via down-regulation of inflammatory signaling in mice.J Ethnopharmacol. 2013 Jul 30;148(3):835-41. doi: 10.1016/j.jep.2013.05.020. Epub 2013 Jun 6. J Ethnopharmacol. 2013. PMID: 23747643
-
Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review.Biomed Pharmacother. 2022 Sep;153:113256. doi: 10.1016/j.biopha.2022.113256. Epub 2022 Jul 14. Biomed Pharmacother. 2022. PMID: 36076615 Review.
-
The Modulatory Bioeffects of Pomegranate (Punica granatum L.) Polyphenols on Metabolic Disorders: Understanding Their Preventive Role against Metabolic Syndrome.Nutrients. 2023 Nov 22;15(23):4879. doi: 10.3390/nu15234879. Nutrients. 2023. PMID: 38068738 Free PMC article. Review.
Cited by
-
Salvianolic Acid A Protects against Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Neutrophil NETosis.Oxid Med Cell Longev. 2022 Jul 21;2022:7411824. doi: 10.1155/2022/7411824. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35910849 Free PMC article.
-
A Study of the Disruptive Effect of the Acetate Fraction of Punica granatum Extract on Cryptococcus Biofilms.Front Microbiol. 2021 Jan 18;11:568258. doi: 10.3389/fmicb.2020.568258. eCollection 2020. Front Microbiol. 2021. PMID: 33537008 Free PMC article.
-
EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response.Sci Rep. 2021 May 26;11(1):11014. doi: 10.1038/s41598-021-90398-x. Sci Rep. 2021. PMID: 34040072 Free PMC article.
-
Indian Medicinal Plants and Formulations and Their Potential Against COVID-19-Preclinical and Clinical Research.Front Pharmacol. 2021 Mar 2;11:578970. doi: 10.3389/fphar.2020.578970. eCollection 2020. Front Pharmacol. 2021. PMID: 33737875 Free PMC article. Review.
-
Unlocking the therapeutic treasure of pomegranate leaf: A comprehensive review on phytochemical compounds, health benefits, and future prospects.Food Chem X. 2024 Jun 22;23:101587. doi: 10.1016/j.fochx.2024.101587. eCollection 2024 Oct 30. Food Chem X. 2024. PMID: 39036478 Free PMC article. Review.
References
-
- Bittencourt-Mernak M, Pinheiro N, Santana F, Guerreiro M, Saraiva-Romanholo B, Grecco S, et al. . Prophylactic and therapeutic treatment with the flavonone sakuranetin ameliorates LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. (2017) 312:L217–30. 10.1152/ajplung.00444.2015 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

