Anti-CD19 Chimeric Antigen Receptor T Cells in Combination With Nivolumab Are Safe and Effective Against Relapsed/Refractory B-Cell Non-hodgkin Lymphoma
- PMID: 31482064
- PMCID: PMC6709654
- DOI: 10.3389/fonc.2019.00767
Anti-CD19 Chimeric Antigen Receptor T Cells in Combination With Nivolumab Are Safe and Effective Against Relapsed/Refractory B-Cell Non-hodgkin Lymphoma
Abstract
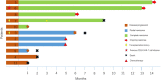
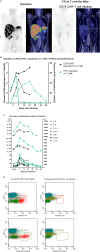
Chimeric antigen receptor (CAR) T cells are emerging as a novel treatment for patients with refractory/relapsed B-cell non-Hodgkin lymphoma (B-NHL), and combination with PD1 inhibitors may further improve the efficacy of anti-CD19 CAR (CD19 CAR)-T cells in the treatment of lymphomas. In a single-center study, we evaluated the safety and efficacy of a combination therapy with CD19 CAR-T cells and an anti-PD-1 antibody (nivolumab) in patients with relapsed/refractory B-NHL. A total of 11 patients with refractory/relapsed B-NHL were recruited and subsequently received CD19 CAR-T cells and nivolumab. The primary end points were safety and feasibility. The infusions were safe, and no dose-limiting toxicities occurred. Grade 1 or 2 cytokine release syndrome (CRS) was observed in 25% (3/11) and 50% (6/11) of the patients, respectively, and only one patient (1/11) experienced neurotoxicity. The objective response rate (ORR) and complete response (CR) rate were 81.81% (9/11) and 45.45% (5/11), respectively. The median follow-up time was 6 (1~15) months. The median progression-free survival (PFS) time was 6 months (1~14 months), and 3 patients continued to have a response at the time of this writing. Our study demonstrated that the combination of CD19 CAR-T cells and nivolumab was feasible and safe and mediated potent anti-lymphoma activity, which should be examined further in prospective clinical trials in refractory/relapsed B-NHL.
Keywords: B-cell non-Hodgkin lymphoma (B-NHL); anti-CD19 chimeric antigen receptor T cells; combination; immune check point blocade; safe and effective.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

