Pushing the Ligand Efficiency Metrics: Relative Group Contribution (RGC) Model as a Helpful Strategy to Promote a Fragment "Rescue" Effect
- PMID: 31482085
- PMCID: PMC6710606
- DOI: 10.3389/fchem.2019.00564
Pushing the Ligand Efficiency Metrics: Relative Group Contribution (RGC) Model as a Helpful Strategy to Promote a Fragment "Rescue" Effect
Abstract
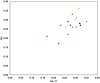
The ligand efficiency (LE) indexes have long been used as decision-making criteria in drug discovery and development. However, in the context of fragment-based drug design (FBDD), these metrics often exhibit a strong emphasis toward the selection of highly efficient "core" fragments for potential optimization, which are not usually considered as parts of a larger molecule with a size typical for a drug. In this study, we present a relative group contribution (RGC) model intended to predict the efficiency of a drug-sized compound in terms of its component fragments. This model could be useful not only in rapidly predicting all the possible combinations of promising fragments from an earlier hit discovery stage, but also in enabling a relatively low-LE fragment to become part of a drug-sized compound as long as it is "rescued" by other high-LE fragments.
Keywords: drug discovery; fragment library; fragment-based screening; ligand efficiency metrics; property-based design; protein-ligand interactions; structure-activity relationship.
Figures
 /
/ LDHA
LDHA  Replication Protein A (RPA)
Replication Protein A (RPA)  /
/ Blood coagulation factor Xa
Blood coagulation factor Xa  Bcl-2
Bcl-2  DOT1L
DOT1L  Hsp90
Hsp90  /
/ Pantothenate synthetase (PtS)
Pantothenate synthetase (PtS)  Blood coagulation factor XIa
Blood coagulation factor XIa  CK2
CK2  BACE1
BACE1  Endothiapepsin (Epn)
Endothiapepsin (Epn)  Bcl-xL
Bcl-xL
 PKM2].
PKM2].References
-
- Albert J. S., Blomberg N., Breeze A., Brown A., Burrows J., Edwards P., et al. (2007). An integrated approach to fragment-based lead generation: philosophy, strategy and case studies from AstraZeneca's Drug Discovery Programmes. Curr. Top. Med. Chem. 7, 1600–1629. 10.2174/156802607782341091 - DOI - PubMed
-
- Cherry M., Mitchell T. (2008). Introduction to fragment-based drug discovery, in Fragment-Based Drug Discovery, eds Zartler E. R., Shapiro M. J. 1–32. 10.1002/9780470721551.ch1 - DOI
-
- Costantino L., Barlocco D. (2018). Designing Approaches to Multitarget Drugs, in Drug Selectivity: An Evolving Concept in Medicinal Chemistry, 161–205.
LinkOut - more resources
Full Text Sources

