A phase II study of the L19IL2 immunocytokine in combination with dacarbazine in advanced metastatic melanoma patients
- PMID: 31482307
- PMCID: PMC11028321
- DOI: 10.1007/s00262-019-02383-z
A phase II study of the L19IL2 immunocytokine in combination with dacarbazine in advanced metastatic melanoma patients
Abstract
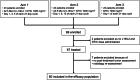
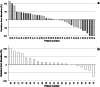
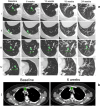
Engineered cytokine products represent promising agents for the treatment of immunogenic tumors, such as malignant melanoma, in addition to immune checkpoint inhibitors. Here we describe the results of a controlled, randomized phase II clinical trial, aimed at assessing the therapeutic potential of L19IL2, a fully human fusion protein consisting of the L19 antibody specific to the alternatively spliced extra-domain B of fibronectin, fused to human interleukin-2 in advanced metastatic melanoma. In one arm, patients received dacarbazine (DTIC; 1000 mg/m2 of body surface on day 1 of 21-day cycles) as single agent, while in two other arms L19IL2 (22.5 million international units of IL2 equivalents) was added, based on two different schedules of administration. In total, 69 patients with stage IV melanoma were enrolled (24 in the dacarbazine arm, 23 and 22 in the other combination arms, respectively) and 67 received treatment. Analyses of efficacy results show a statistically significant benefit in terms of overall response rate and median progression-free survival for patients receiving L19IL2 in combination with DTIC, compared to DTIC as single agent. In light of these results, further clinical investigations with L19IL2 (alone or in combination with other agents) are warranted.
Keywords: Dacarbazine; Immunocytokine; L19IL2; Phase II study; Stage IV melanoma.
Conflict of interest statement
Benjamin Weide has received speaker or advisory board honoraria from Amgen, CureVac, Philogen, Novartis as well as research funding from Bristol-Myers Squibb (BMS), Merck, Sharp & Dohme (MSD) and Philogen. Paolo Ascierto reports grants and personal fees from BMS, grants and personal fees from Roche-Genentech, personal fees from MSD, grants and personal fees from Array, personal fees from Novartis, personal fees from Merck Serono, personal fees from Pierre Fabre, personal fees from Incyte, personal fees from Genmab, personal fees from NewLink Genetics, personal fees from Medimmune, personal fees from AstraZeneca, personal fees from Syndax, personal fees from Sun Pharma, personal fees from Sanofi, personal fees from Idera, personal fees from Ultimovacs, personal fees from Sandoz, personal fees from Immunocore, personal fees from 4SC, outside the submitted work; Jürgen C. Becker has received speaker honoraria from Amgen, Merck Serono, and Pfizer, advisory board honoraria from Amgen, CureVac, eTheRNA, Lytix, Merck Serono, Novartis, Rigontec, and Takeda as well as research funding from Alcedis, Boehringer Ingelheim, BMS and Merck Serono; he also received travel support from 4SC and Incyte; Axel Hauschild received clinical trial support, speaker´s honoraria, or consultancy fees from the following companies: Amgen, BMS, Merck Serono, MSD, Novartis, Philogen, Pierre Fabre, Provectus, Regeneron, Roche, OncoSec, Sanofi-Genzyme, and Sun Pharma; Reinhard Dummer reports intermittent, project focused consulting and/or advisory relationships with Novartis, MSD, BMS, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome outside the submitted work; Giuliano Elia is an employee of Philochem AG, a company of the Philogen group; Dario Neri is shareholder and Board Member of Philogen S.p.A.; Claus Garbe reports personal fees from Philogen, during the conduct of the study; personal fees from Amgen, personal fees from MSD, grants and personal fees from Novartis, personal fees from NeraCare, grants and personal fees from BMS, personal fees from Philogen, grants and personal fees from Roche, grants and personal fees from Sanofi, outside the submitted work. All other authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

