Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine
- PMID: 31483291
- PMCID: PMC6763255
- DOI: 10.1172/JCI128531
Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine
Abstract
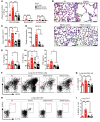
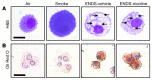
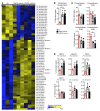
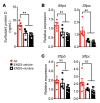
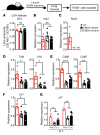
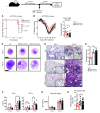
Electronic nicotine delivery systems (ENDS) or e-cigarettes have emerged as a popular recreational tool among adolescents and adults. Although the use of ENDS is often promoted as a safer alternative to conventional cigarettes, few comprehensive studies have assessed the long-term effects of vaporized nicotine and its associated solvents, propylene glycol (PG) and vegetable glycerin (VG). Here, we show that compared with smoke exposure, mice receiving ENDS vapor for 4 months failed to develop pulmonary inflammation or emphysema. However, ENDS exposure, independent of nicotine, altered lung lipid homeostasis in alveolar macrophages and epithelial cells. Comprehensive lipidomic and structural analyses of the lungs revealed aberrant phospholipids in alveolar macrophages and increased surfactant-associated phospholipids in the airway. In addition to ENDS-induced lipid deposition, chronic ENDS vapor exposure downregulated innate immunity against viral pathogens in resident macrophages. Moreover, independent of nicotine, ENDS-exposed mice infected with influenza demonstrated enhanced lung inflammation and tissue damage. Together, our findings reveal that chronic e-cigarette vapor aberrantly alters the physiology of lung epithelial cells and resident immune cells and promotes poor response to infectious challenge. Notably, alterations in lipid homeostasis and immune impairment are independent of nicotine, thereby warranting more extensive investigations of the vehicle solvents used in e-cigarettes.
Keywords: Immunology; Inflammation; Innate immunity.
Conflict of interest statement
Figures



Comment in
-
Less burn, more fat: electronic cigarettes and pulmonary lipid homeostasis.J Clin Invest. 2019 Oct 1;129(10):4077-4079. doi: 10.1172/JCI131336. J Clin Invest. 2019. PMID: 31483292 Free PMC article.
-
Scientists chase cause of mysterious vaping illness as death toll rises.Nature. 2019 Oct;574(7778):303-304. doi: 10.1038/d41586-019-03033-1. Nature. 2019. PMID: 31616092 No abstract available.
References
-
- McCarthy M. E-cigarettes are major threat to young people’s health, says US surgeon general. BMJ. 2016;355:i6652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

