Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer
- PMID: 31483485
- PMCID: PMC6856402
- DOI: 10.1002/cncr.32445
Real-world outcomes of sipuleucel-T treatment in PROCEED, a prospective registry of men with metastatic castration-resistant prostate cancer
Abstract
Background: The large registry, PROVENGE Registry for the Observation, Collection, and Evaluation of Experience Data (PROCEED)(NCT01306890), evaluated sipuleucel-T immunotherapy for asymptomatic/minimally symptomatic metastatic castration-resistant prostate cancer (mCRPC).
Methods: PROCEED enrolled patients with mCRPC receiving 3 biweekly sipuleucel-T infusions. Assessments included overall survival (OS), serious adverse events (SAEs), cerebrovascular events (CVEs), and anticancer interventions (ACIs). Follow-up was for ≥3 years or until death or study withdrawal.
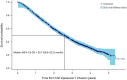
Results: In 2011-2017, 1976 patients were followed for 46.6 months (median). The median age was 72 years, and the baseline median prostate-specific antigen level was 15.0 ng/mL; 86.7% were white, and 11.6% were African American. Among the patients, 1902 had 1 or more sipuleucel-T infusions. The median OS was 30.7 months (95% confidence interval [CI], 28.6-32.2 months). Known prognostic factors were independently associated with OS in a multivariable analysis. Among the 1255 patients who died, 964 (76.8%) died of prostate cancer (PC) progression. The median time from the first infusion to PC death was 42.7 months (95% CI, 39.4-46.2 months). The incidence of sipuleucel-T-related SAEs was 3.9%. The incidence of CVEs was 2.8%, and the rate per 100 person-years was 1.2 (95% CI, 0.9-1.6). The CVE incidence among 11,972 patients with mCRPC from the Surveillance, Epidemiology, and End Results-Medicare database was 2.8%; the rate per 100 person-years was 1.5 (95% CI, 1.4-1.7). One or more ACIs (abiraterone, enzalutamide, docetaxel, cabazitaxel, or radium 223) were received by 77.1% of the patients after sipuleucel-T; 32.5% and 17.4% of the patients experienced 1- and 2-year treatment-free intervals, respectively.
Conclusions: PROCEED provides contemporary survival data for sipuleucel-T-treated men in a real-world setting of new life-prolonging agents, which will be useful in discussing treatment options with patients and in powering future trials with sipuleucel-T. The safety and tolerability of sipuleucel-T in PROCEED were consistent with previous findings.
Keywords: immunotherapy; overall survival; prostate cancer; safety.
© 2019 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Conflict of interest statement
Celestia S. Higano has served in an advisory role for Aptevo, Asana, Astellas, Bayer, Blue Earth Diagnostics, Churchill Pharma, Clovis Oncology, Dendreon, Endocyte, Ferring, Medivation, Orion Corporation, and Pfizer; she has also participated in sponsored research for Aptevo, Bayer, Aragon Pharma, Astellas, AstraZeneca, Dendreon, Genentech, Hoffman‐LaRoche, Medivation, Sanofi, and Pfizer, and her spouse was in a leadership role for CTI Biopharma. Andrew J. Armstrong has received grants and personal fees from Dendreon, Pfizer/Astellas, Janssen, Bayer, and Sanofi‐Aventis during this study as well as grants from Novartis, Gilead, Bristol‐Myers Squibb, and Genentech/Roche outside the submitted work. A. Oliver Sartor has served as a consultant for and received personal fees from Advanced Accelerator Applications, Astellas, AstraZeneca, Bavarian‐Nordic, Bayer, Bellicum, Blue Earth Diagnostics, Celgene, Constellation, Dendreon, EMD Serono, Endocyte, Johnson & Johnson, Bristol‐Myers Squibb, Myovant, Pfizer, Progenics, Sanofi, Teva, and Hinova during this study; he has also received grants from AstraZeneca, Bayer, Constellation, Dendreon, Endocyte, Johnson & Johnson, Bristol‐Myers Squibb, Progenics, Sanofi, Innocrin, Invitae, Merck, Roche, and Sotio. Philip W. Kantoff has received personal fees from Astellas, Bayer, Bellicum, BIND Biosciences, Bavarian Nordic Immunotherapies, DRGT, Genentech/Roche, Ipsen Pharmaceuticals, Janssen, Metamark, Merck, Millennium/Prometrika, MTG, Omnitura, OncoCell MDx, OncoGenex, Progenity, Sanofi, Tarveda Pharmaceuticals, Thermo Fisher, GE Healthcare, Context Therapeutics, New England Research Institutes, SEER Biosciences, and Placon; he also has investment interests in DRGT, Tarveda Pharmaceuticals, Context Therapeutics, SEER Biosciences, and Placon. Christopher M. Pieczonka has received personal fees as a consultant for Dendreon, Bayer, Janssen, and Pfizer and as an investigator for Dendreon, Bayer, Janssen, Pfizer, Merck, AstraZeneca, Taiho, Innocrin, and Myovant outside the submitted work. David F. Penson has received personal fees from Dendreon and Janssen as well as a grant from the Vanderbilt University Research Center. Neal D. Shore has served as a consultant for and received personal fees from Ferring, Bayer, Amgen, Janssen, Dendreon, Tolmar, Astellas, Pfizer, AstraZeneca, Genentech/Roche, Myovant Sciences, Merck, Bristol Meyers Squibb, and Nymox outside the submitted work. Raoul S. Concepcion has served in an advisory role for Dendreon and received personal fees outside the submitted work. David I. Quinn has been involved in payments to the University of Southern California for trial conduct with Dendreon; he has also acted as an advisor for and received personal fees from Dendreon, Bayer, Janssen, Pfizer, Astellas, Genzyme, Clovis, and AstraZeneca. Vahan Kassabian has served as a consultant or speaker for Dendreon, Amgen, Astellas, Pfizer, Janssen, Bayer, UroGPO, Tolmar, and Genomic Health outside the submitted work and is a shareholder of UroGPO. Matt Harmon reports stock ownership in Amgen. Robert C. Tyler has been an employee of Janssen, Dendreon, Medivation, Pfizer, and Innocrin. Nancy N. Chang was a full‐time employee of Dendreon at the time of the analyses and drafting of this manuscript. Hong Tang was a full‐time employee of Dendreon at the time of the analyses and drafting of the manuscript; is a nonexecutive director of OnQuality Pharmaceuticals; and owns stock in BeiGene, Nektar, Sangamo Therapeutics, Tesaro, Verastem, Editas Medicine, and CVS Health Corporation. Matthew R. Cooperberg has received personal fees from Dendreon in relation to a PROCEED trial steering committee and has served in an advisory or consultancy role for Bayer, MDx Health, and Myriad Genetics; he has also participated in a registry steering committee for Astellas. The other authors made no disclosures.
Figures
Comment in
-
Reply to Potential underestimation of cerebrovascular events in the PROVENGE Registry for the Observation, Collection, and Evaluation of Experience Data.Cancer. 2020 Jun 15;126(12):2935-2937. doi: 10.1002/cncr.32785. Epub 2020 Mar 10. Cancer. 2020. PMID: 32154908 Free PMC article. No abstract available.
-
Potential underestimation of cerebrovascular events in the PROVENGE Registry for the Observation, Collection, and Evaluation of Experience Data.Cancer. 2020 Jun 15;126(12):2934-2935. doi: 10.1002/cncr.32786. Epub 2020 Mar 10. Cancer. 2020. PMID: 32154909 No abstract available.
Similar articles
-
Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry.Prostate Cancer Prostatic Dis. 2020 Sep;23(3):517-526. doi: 10.1038/s41391-020-0213-7. Epub 2020 Feb 28. Prostate Cancer Prostatic Dis. 2020. PMID: 32111923 Free PMC article.
-
Outcomes in men with metastatic castration-resistant prostate cancer who received sipuleucel-T and no immediate subsequent therapy: experience at Dana Farber and in the PROCEED Registry.Prostate Cancer Prostatic Dis. 2022 Feb;25(2):314-319. doi: 10.1038/s41391-022-00493-x. Epub 2022 Feb 10. Prostate Cancer Prostatic Dis. 2022. PMID: 35145218 Clinical Trial.
-
Real-World Effectiveness of Sipuleucel-T on Overall Survival in Men with Advanced Prostate Cancer Treated with Androgen Receptor-Targeting Agents.Adv Ther. 2022 Jun;39(6):2515-2532. doi: 10.1007/s12325-022-02085-6. Epub 2022 Mar 30. Adv Ther. 2022. PMID: 35352309 Free PMC article.
-
Sipuleucel-T for the Treatment of Metastatic Hormone-Relapsed Prostate Cancer: A NICE Single Technology Appraisal; An Evidence Review Group Perspective.Pharmacoeconomics. 2015 Nov;33(11):1187-94. doi: 10.1007/s40273-015-0296-5. Pharmacoeconomics. 2015. PMID: 26017401 Review.
-
Sipuleucel-T (Provenge®) for castration-resistant prostate cancer.BJU Int. 2012 Jul;110(2 Pt 2):E99-104. doi: 10.1111/j.1464-410X.2011.10790.x. Epub 2011 Dec 16. BJU Int. 2012. PMID: 22177289 Review.
Cited by
-
Current progress in the development of prophylactic and therapeutic vaccines.Sci China Life Sci. 2023 Apr;66(4):679-710. doi: 10.1007/s11427-022-2230-4. Epub 2022 Dec 2. Sci China Life Sci. 2023. PMID: 36469218 Free PMC article. Review.
-
Progression in immunotherapy for advanced prostate cancer.Front Oncol. 2023 Feb 28;13:1126752. doi: 10.3389/fonc.2023.1126752. eCollection 2023. Front Oncol. 2023. PMID: 36925917 Free PMC article. Review.
-
Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry.Prostate Cancer Prostatic Dis. 2020 Sep;23(3):517-526. doi: 10.1038/s41391-020-0213-7. Epub 2020 Feb 28. Prostate Cancer Prostatic Dis. 2020. PMID: 32111923 Free PMC article.
-
Androgen receptor activity in T cells limits checkpoint blockade efficacy.Nature. 2022 Jun;606(7915):791-796. doi: 10.1038/s41586-022-04522-6. Epub 2022 Mar 23. Nature. 2022. PMID: 35322234 Free PMC article.
-
Immune-Checkpoint Blockade Enhances 225Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer.J Nucl Med. 2021 Feb;62(2):228-231. doi: 10.2967/jnumed.120.246041. Epub 2020 Jul 9. J Nucl Med. 2021. PMID: 32646877 Free PMC article.
References
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel‐T immunotherapy for castration‐resistant prostate cancer. N Engl J Med. 2010;363:411‐422. - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Prostate Cancer: Version 1.2018. Published February 14, 2018. Accessed November 21, 2018. https://www.nccn.org/professionals/physician_gls/default.aspx
-
- Cookson MS, Roth BJ, Dahm P, et al. Castration‐resistant prostate cancer: AUA guidelines 2018. Accessed December 11, 2018. http://www.auanet.org/guidelines/prostate-cancer-castration-resistant-(2...